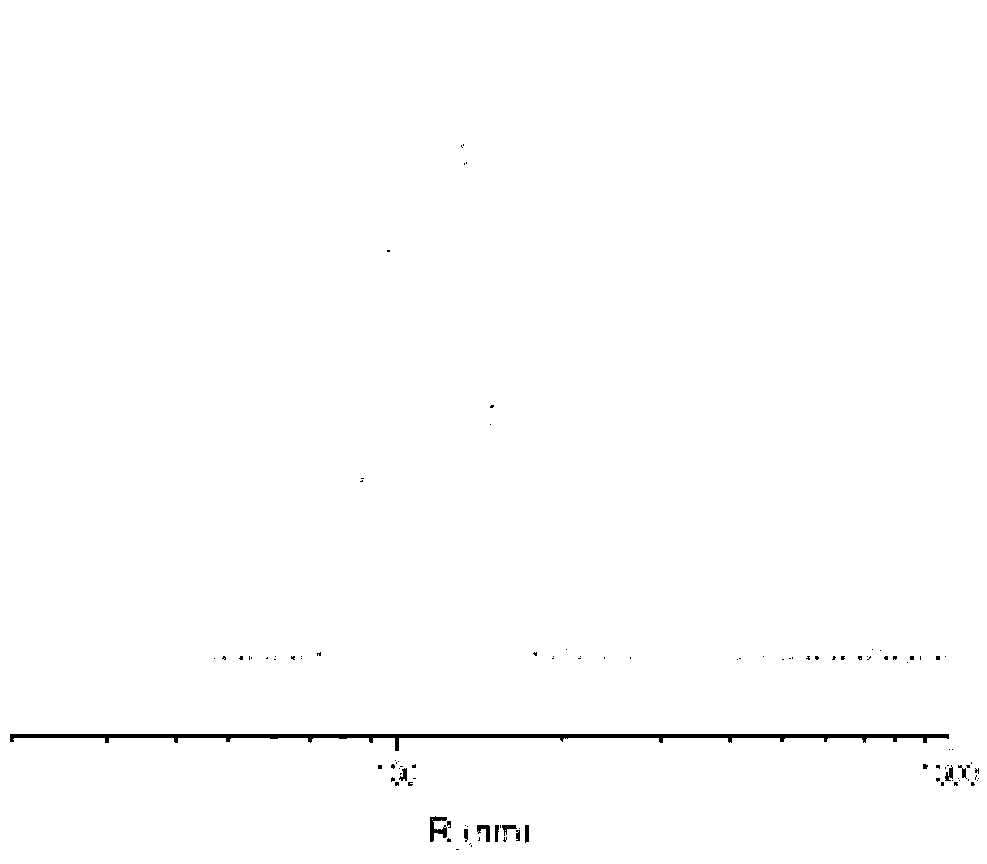
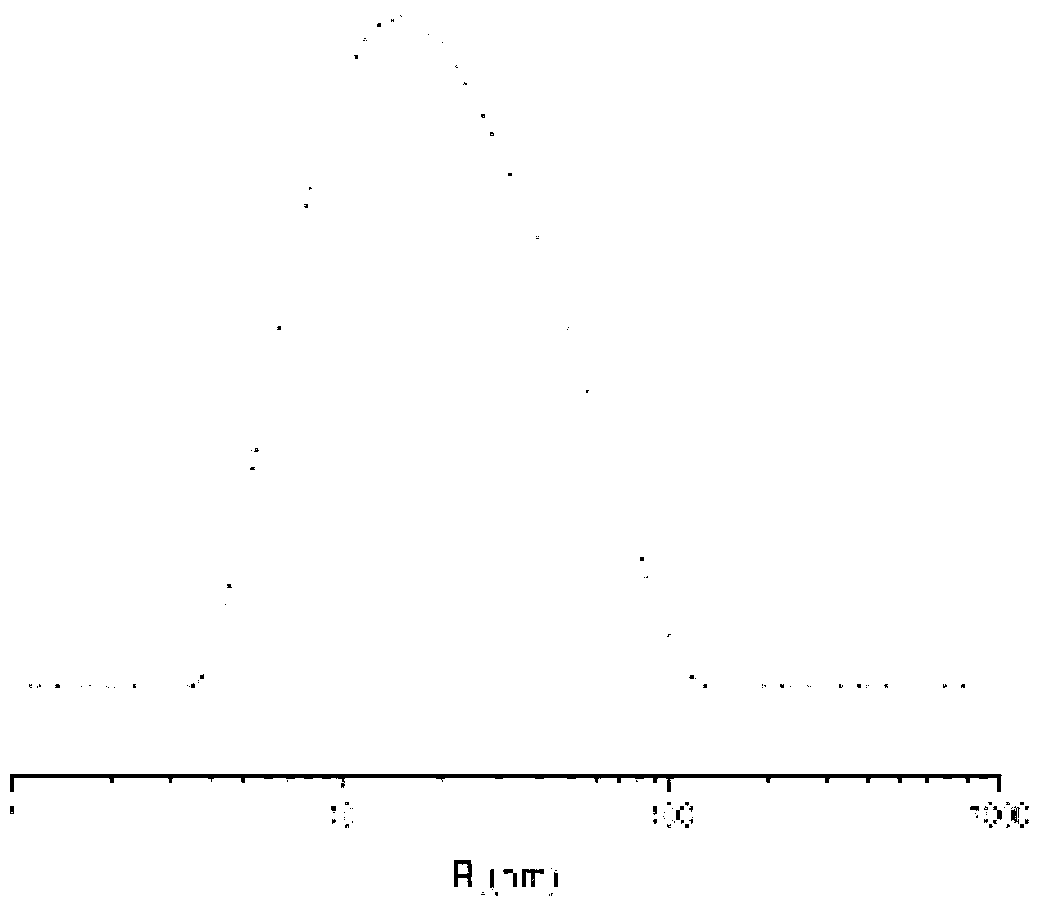
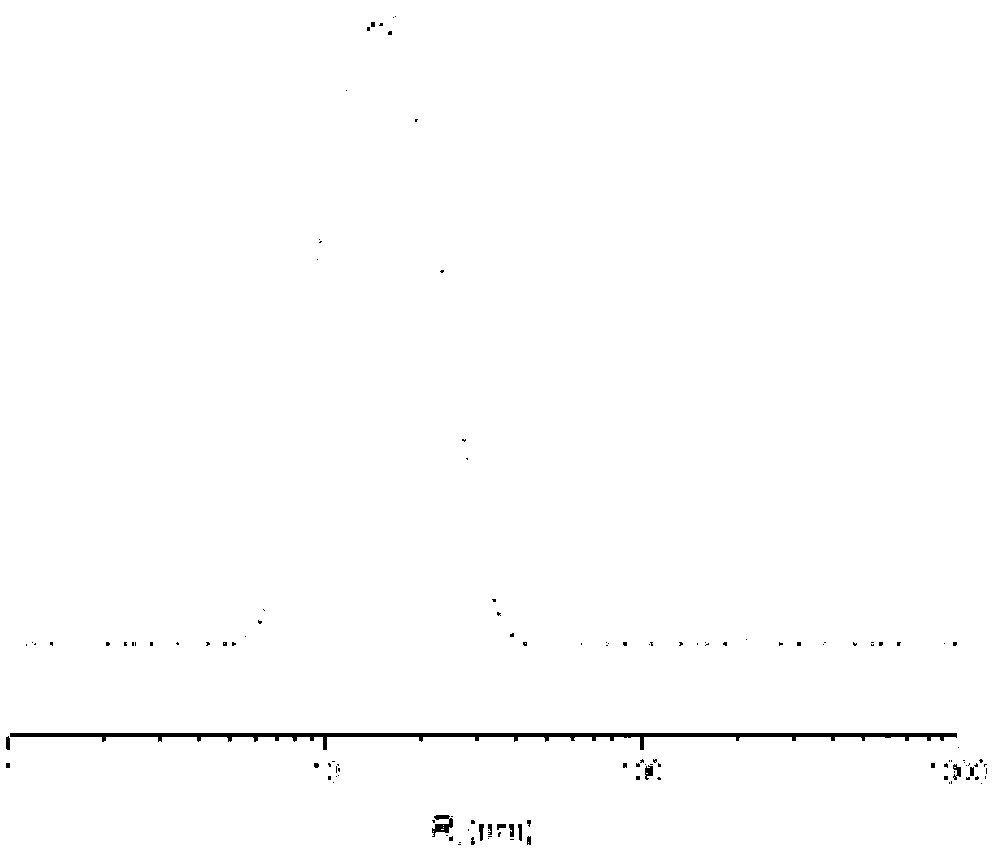
Cisplatin complex and preparation method thereof
A complex, cisplatin technology, applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. It can avoid the sudden release of cisplatin, promote the release and improve the stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0068] The present invention also provides a preparation method of a cisplatin complex, which comprises the following steps: a complex reaction occurs between cisplatin and a polymer having the structure of formula (I) in an aqueous medium to generate a cisplatin complex;
[0069]
[0070] In formula (I), R 1 Independently selected from C2~C10 straight chain alkyl, C3~C10 branched chain alkyl, phenyl or R'-CO-, R' independently selected from C2~C10 straight chain alkyl, C3~C10 branched alkyl or phenyl, R 1 Preferably C3-C8 straight-chain alkyl, C4-C8 branched-chain alkyl, phenyl, more preferably C6 alkyl;
[0071] R 2 Independently selected from H atom, C1~C20 alkyl or substituted C1~C20 alkyl, preferably H atom, C1~C10 alkyl or substituted C1~C10 alkyl, the substituted alkyl The substituents are selected from one or more of ketal, aldol, hydroxyl, aldehyde, amino, sulfhydryl and sugar residues;
[0072] R 3 independently selected from H atoms or cations, preferably H ...
Embodiment 1
[0096] 10g of γ-benzyl-L-glutamic acid was added to 100ml of anhydrous tetrahydrofuran under the condition of dry inert gas, fully reacted under the action of 6.5g of triphosgene, then petroleum ether was added to settle to obtain a solid, weighing After crystallization and drying, 7.85g of γ-benzyl-L-glutamic acid-N-carboxylic anhydride (BLG-NCA) monomer was finally obtained; under the condition of inert gas, the dimethylformamide (DMF) of n-hexylamine Add the solution to the DMF solution of BLG-NCA, the ratio of n-hexylamine to γ-benzyl-L-glutamic acid-N-carboxylic acid anhydride (BLG-NCA) is 1:160, add ether to settle after reaction, and filter Obtain a solid, and after drying, obtain a glutamic acid copolymer with a benzyl protecting group with a degree of polymerization of 160; after deprotection, obtain a glutamic acid copolymer with a structure of formula (II) with a degree of polymerization of 160, denoted as P(Glu) 160 .
[0097] Add 1.010g of P(Glu) prepared above t...
Embodiment 2
[0100] 10g of γ-benzyl-L-glutamic acid was added to 100ml of anhydrous tetrahydrofuran under the condition of dry inert gas, fully reacted under the action of 6.5g of triphosgene, then petroleum ether was added to settle to obtain a solid, weighing After crystallization and drying, 7.34g of γ-benzyl-L-glutamic acid-N-carboxylic anhydride (BLG-NCA) monomer was finally obtained; under the condition of inert gas, the dimethylformamide (DMF) solution of n-hexylamine Added to the DMF solution of BLG-NCA, the mass ratio of n-hexylamine and γ-benzyl-L-glutamic acid-N-carboxylic acid anhydride (BLG-NCA) was 1:160, after the reaction, adding ether to settle, and filtered to obtain Solid, after drying, obtain a glutamic acid copolymer with a benzyl protecting group with a degree of polymerization of 160; after deprotection, obtain a glutamic acid copolymer with a structure of formula (II) with a degree of polymerization of 160, denoted as P(Glu) 160 .
[0101] Add the 1.012g P(Glu) pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com