Fluorescent sensor for measuring picric acid content and preparation method thereof
A picric acid and chemical sensor technology, applied in the field of fluorescence sensor, can solve the problems of high detection limit of spectrophotometry, shortened service life of chromatographic column, many operation steps, etc., and achieves the effects of online measurement, simple structure and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Synthetic steps of allyl tetraiodofluorescein The synthetic route in the reference (Anal. Chim. Acta, 1997: V342 207-213), the specific steps are as follows:
[0017] Sodium tetraiodofluorescein (compound A) and bromopropene (compound B) with a molar ratio of 1:3 were stirred in N,N-dimethylformamide at 80°C for 3 hours to fully react, After the reaction was completed, the N,N-dimethylformamide was removed by rotary evaporation, and the obtained solid was dissolved in water, and 0.2mol l -1 The hydrochloric acid was adjusted to acidity, resulting in a red precipitate, which was filtered, dried, and recrystallized from ethanol. A tetraiodofluorescein derivative (compound C) with a terminal double bond was obtained with a yield of 65%, MS: (M+H) + 877.
[0018]
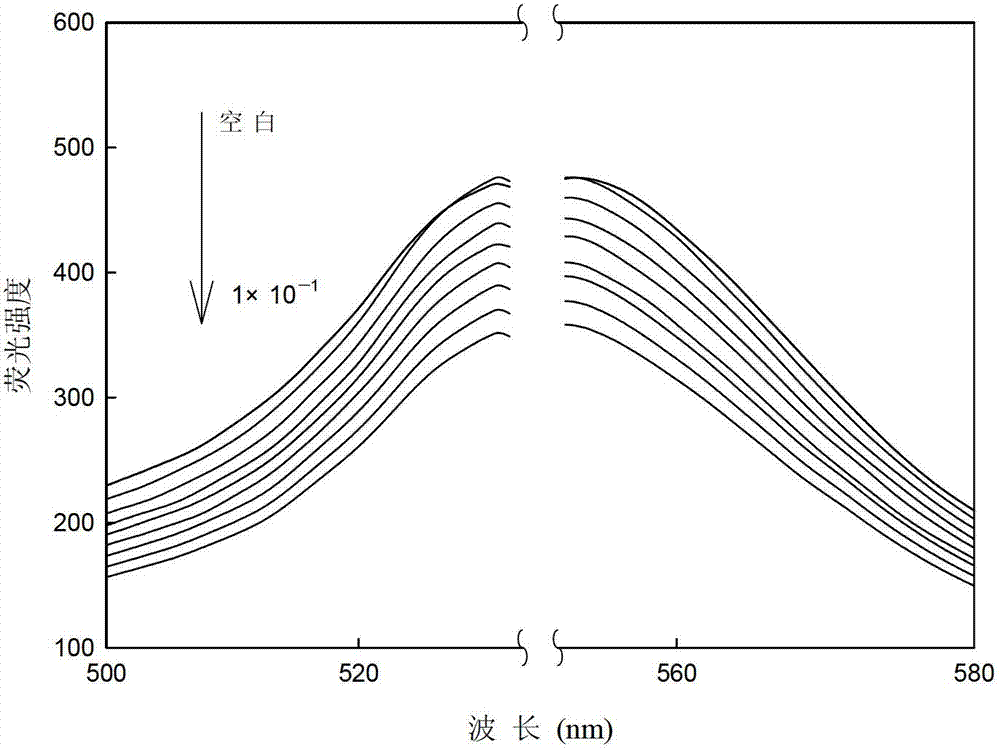
[0019] The nitro group on the aromatic ring in picric acid is a strong electron-withdrawing group, which forms a fluorescent complex with tetraiodofluorescein, resulting in the fluorescence quenching of tetr...
Embodiment 2
[0021] The preparation and determination of the fluorescent sensor include the following steps:
[0022] 1. Silanization of glass slides: Soak quartz glass slides (diameter 12.5mm) in chromic acid lotion for 40 minutes, then soak them in 3% hydrofluoric acid for 30 minutes, and then rinse them with distilled water so that they are no longer stained. There is 3% hydrofluoric acid, then add 10% hydrogen peroxide and soak for 30 minutes. Then rinse with distilled water. Measure 0.2ml propyl methacrylate with a dry pipette, 2ml 0.2mol l -1 The acetic acid-sodium acetate buffer solution with a pH of 3.6 was mixed with 8 ml of distilled water, and stirred for 10 minutes to completely dissolve the propyl methacrylate to prepare a silylation solution. The slides were immersed in this solution for 2-3 hours, then washed with distilled water, and dried at room temperature for later use.
[0023] 2. Preparation of the optode film: Dissolve 15 mg of allyl tetraiodofluorescein in 0.2 ml...
Embodiment 3
[0025] Embodiment 3: Determination of correction equation
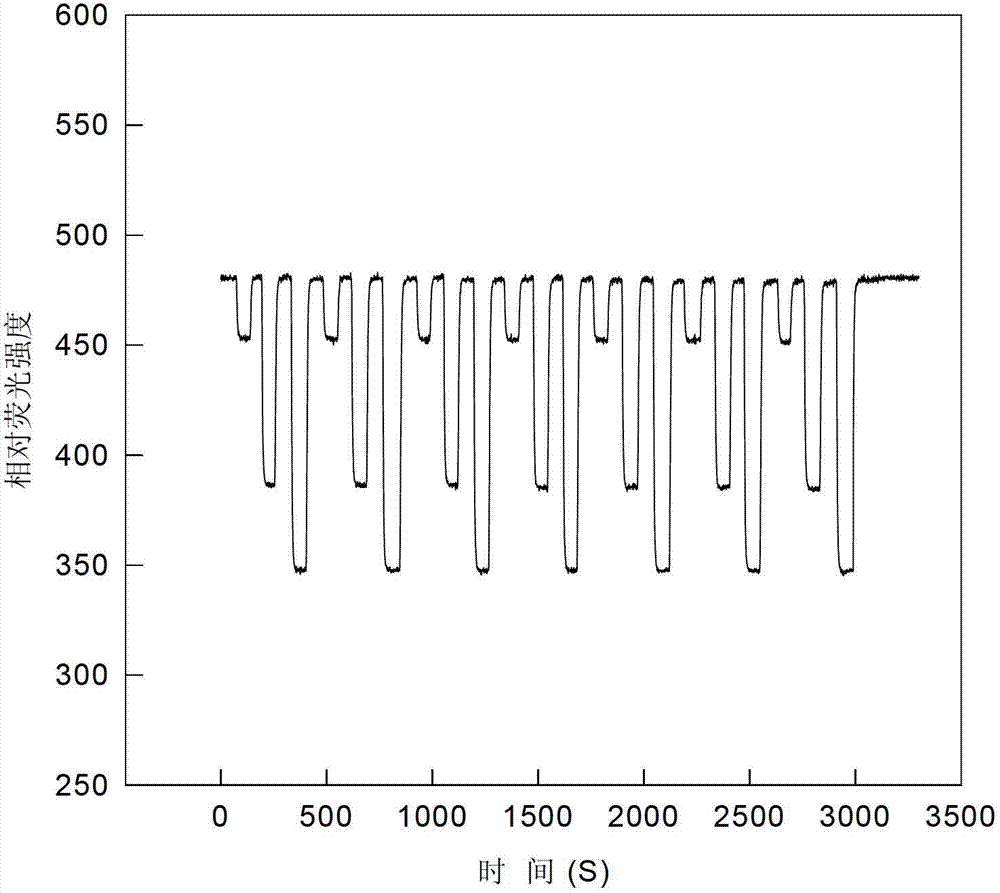
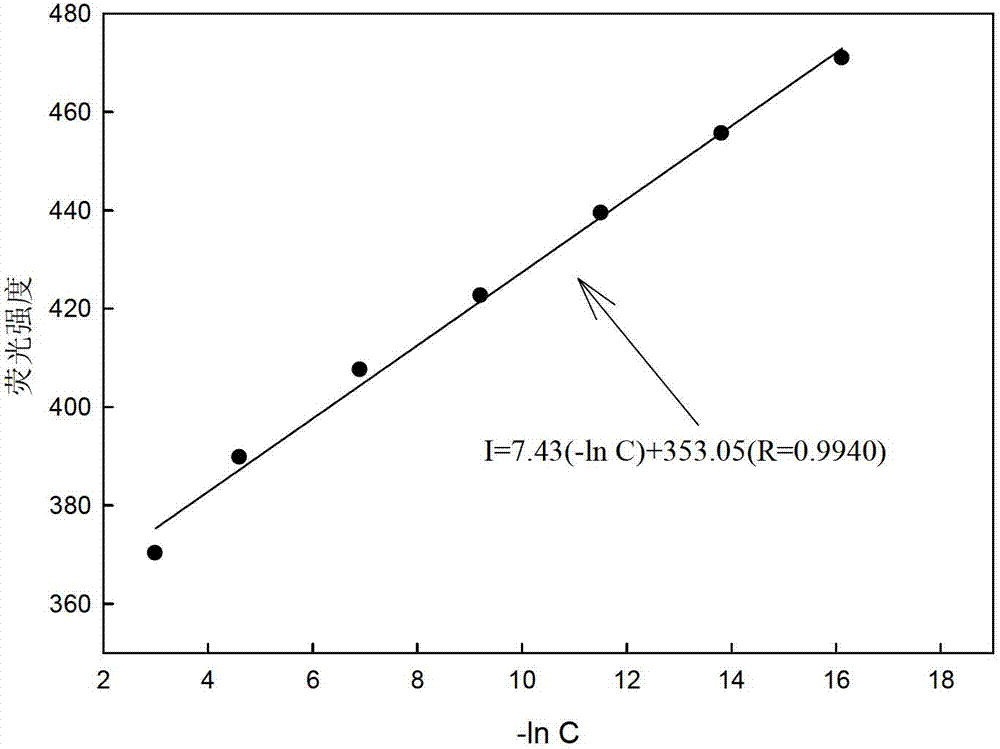
[0026] Different concentrations of picric acid solution (0mol l -1 , 1×10 -7 mol l -1 , 1×10 -6 mol l -1 ,1×10 -5 mol l -1 ,1×10 -4 moll -1 ,1×10 -3 mol l -1 ,1×10 -2 mol l -1 ,0.5×10 -1 moll -1 , 1×10 -1 moll -1 ) are respectively input into the flow cell by the peristaltic pump at a speed of 1.3ml / min, and a stable fluorescence intensity (I) can be obtained after the optode membrane and the solution reach equilibrium. Use Sigmaplot software for straight line fitting, when the picric acid concentration is 1×10 -7 ~0.5×10-1 mol l -1 When between, there is a good linear relationship between -lnC and fluorescence intensity, such as image 3 As shown, the correction equation obtained is: I=7.43(-ln C)+353.05(R=0.9940). The concentration of picric acid is 1×10 -7 ~0.5×10 -1 mol l -1 Between, it can be used as a sensor to measure the quantitative relationship of picric acid.
[0027] Put the sample o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com