Process for production of reduced glutathione
A technology of oxidized glutathione and glutathione, applied in reduction electrolysis, peptide, electrolytic organic production, etc., can solve the problem of incomplete production method of reduced glutathione
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The preparation method of the supersaturated solution of oxidized glutathione is not limited, and can be mentioned: including a method of dissolving oxidized glutathione in an alkaline solution at a high concentration and desalting, including oxidizing glutathione A method of adsorbing a peptide to an ion exchange resin and eluting it in a solution having a high concentration includes a method of concentrating a solution having a concentration not exceeding saturation solubility, and the like.
[0035] The solution used in the anode cell in the present invention is not particularly limited as long as it is a conductive aqueous solution, and there may be mentioned: inorganic acid solutions such as hydrochloric acid and sulfuric acid; organic acid solutions such as acetic acid and propionic acid; agent solution, etc. Regarding the concentration of inorganic acid and organic acid, the conductivity is low at low concentration, and the ion exchange membrane is easily deterio...
Embodiment 1
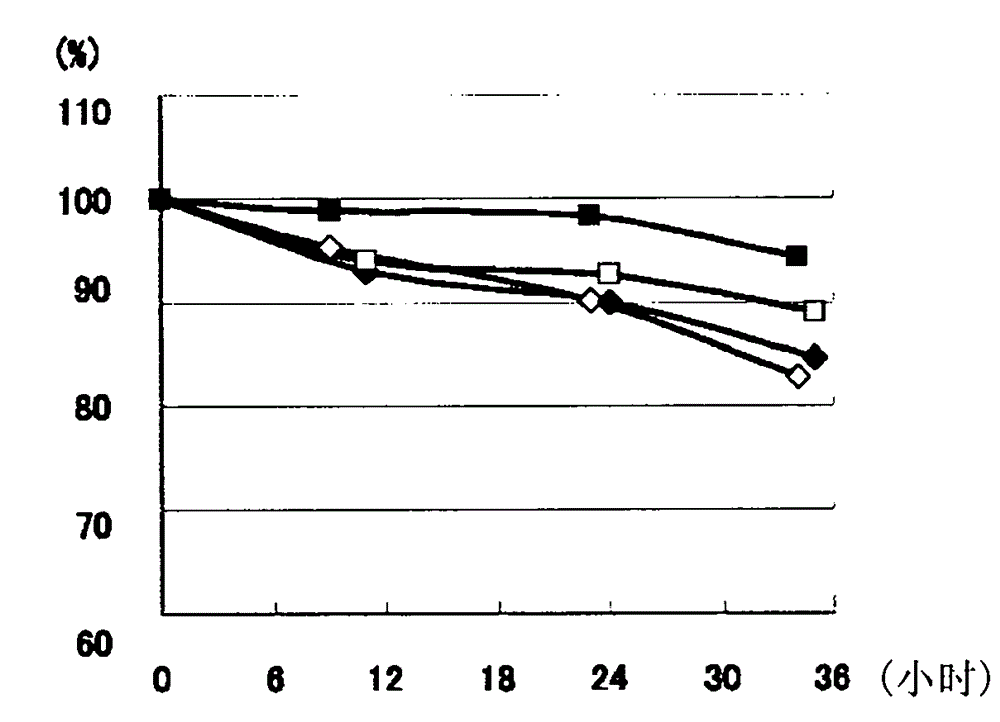
[0043] pH Stability of Reduced Glutathione
[0044] Using reduced glutathione aqueous solution (100 g / L, pH 2.90) and sulfuric acid, reduced glutathione aqueous solutions (100 g / L) adjusted to pH 0.6, 1.2 and 2.0 were produced.
[0045] Each solution was stored at 25° C. for 24 to 36 hours, and the residual amount of reduced glutathione was quantified by high performance liquid chromatography (HPLC) under the following conditions. Such as Figure 1A As shown, it was found that the solution below pH 2.0 had a lower residual rate of reduced glutathione than the solution at pH 2.90. In addition, if Figure 1B As shown, it was found that solutions below pH 2.0 showed a higher increase in impurities due to hydrolysis compared to solutions at pH 2.90. Regarding impurities, the total amount of substances other than reduced glutathione and oxidized glutathione was quantified under the following HPLC conditions, and the concentration (g / L) converted to reduced glutathione was calcula...
Embodiment 2
[0053] Electrolytic Reduction of Oxidized Glutathione Using Sodium Sulfate as Conductive Agent
[0054] An aqueous solution of oxidized glutathione (about 400 g / L) was prepared by adding sodium hydroxide to adjust the pH to 7.0, which was passed through a cation exchange resin for desalination to prepare a supersaturated aqueous solution of oxidized glutathione ( about 350g / L). The solution was diluted, and sodium sulfate was added to prepare an aqueous oxidized glutathione solution (310 g / L) containing 0.75 mol / L sodium sulfate. The pH of the solution was 2.91.
[0055] The electrolyzer used contained anode side (150mL) and cathode side (300mL), and both passed 50cm 2 The cation exchange membrane SELEMION CMT (manufactured by Asahi Glass Co., Ltd.) separated. As an anode, use a 50cm 2 Platinized titanium plate, as cathode, use 50cm 2 zinc plate. The anode tank contained a 0.50 mol / L sulfuric acid solution (140 mL), and the cathode tank contained the oxidized glutathione...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com