An IgY for a PA-MSHA bacterial strain, and preparation method and application thereof
A technology of bacterial strains and biological products, applied in the field of mannose-sensitive Pseudomonas aeruginosa strain IgY and its preparation, can solve the problem that IgY of egg-laying poultry does not have a broad-spectrum antibacterial effect and anti-tumor auxiliary effect, and achieve good activity and specificity sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the method for obtaining the product of egg yolk immunoglobulin with broad-spectrum antibacterial and anticancer
[0032] The antigen of the PA-MSHA strain is obtained through the above process, and an immunized egg, such as a chicken egg, is obtained after injection. The following describes the preparation process of yolk immunoglobulin with broad-spectrum antibacterial and anticancer properties extracted from immunized eggs. The extraction and purification method of the egg yolk immunoglobulin is water dilution method, repeated freezing and thawing method or ammonium sulfate, sodium sulfate, PEG, dextran sulfate, sodium alginate or caprylic acid precipitation or leaching, extraction, concentration and membrane filtration. Methods The antibody is extracted from egg yolk, and then the purified antibody is prepared into any pharmaceutical dosage form including tablets, injections, oral liquids and sprays by adding corresponding auxiliary materials including...
Embodiment 2
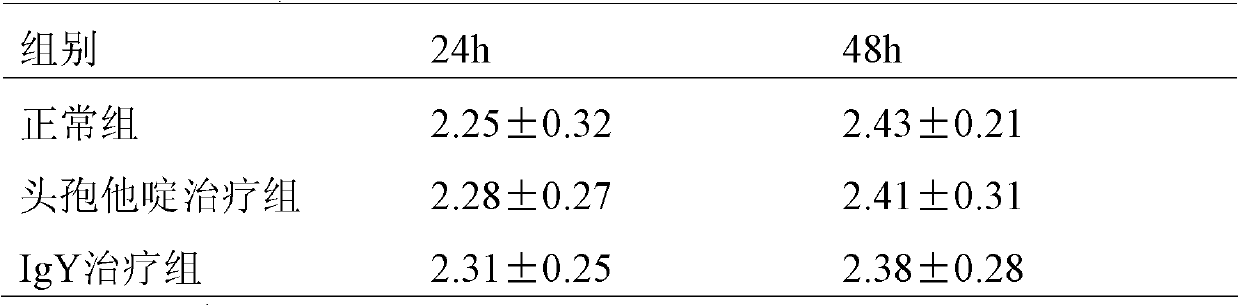
[0040] Embodiment 2: ELISA method detects the potency of egg yolk immunoglobulin
[0041] A 96-well plate was coated with Pseudomonas aeruginosa antigen at 10 μg / ml, overnight at 4°C, washed three times with PBS of pH 7.4 the next day, blocked with 5% calf serum for 12 hours, and air-dried after washing. Dilute the purified egg yolk immunoglobulin (starting from 1:10, a total of 8 concentration groups) with sterile PBS, add 100 μl to each well, and incubate at 37°C for 1 hour; after washing and air-drying, add horseradish peroxidase-labeled sheep Anti-chicken immunoglobulin (HRP-IgG, concentration 1:10 5 ) 100pl, incubated at 37°C for 1h. O-phenylenediamine was developed at 37°C in the dark for 15 minutes, the reaction was terminated with 2 mol / L sulfuric acid, and the A450 value was read on a microplate reader. Set sterile PBS as blank control. The test results confirmed that the titer of the purified egg yolk immunoglobulin was 1:10 5 .
Embodiment 3
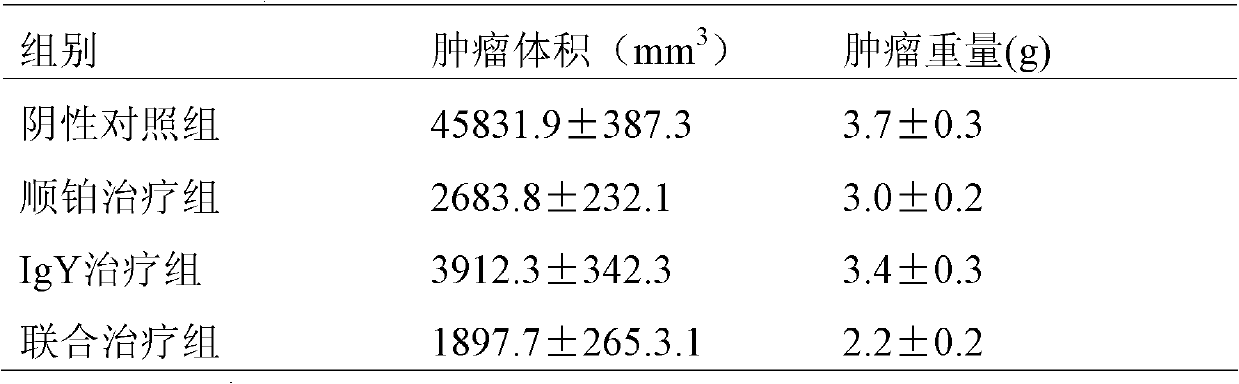
[0042] Embodiment 3: egg yolk immunoglobulin stability test
[0043] Egg yolk immunoglobulin was sterilized by filtration, packed into small test tubes at 10 mg / ml, and stored at -20°C, 4°C and room temperature respectively, and its potency was tested regularly. The experimental results show that the stability of egg yolk immunoglobulin can be maintained for at least 12 months when stored at -20°C; the stability can be maintained for at least 6 months when stored at 4°C; the stability can be maintained for at least 6 months when stored at room temperature. It can be stored for more than 2 months.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com