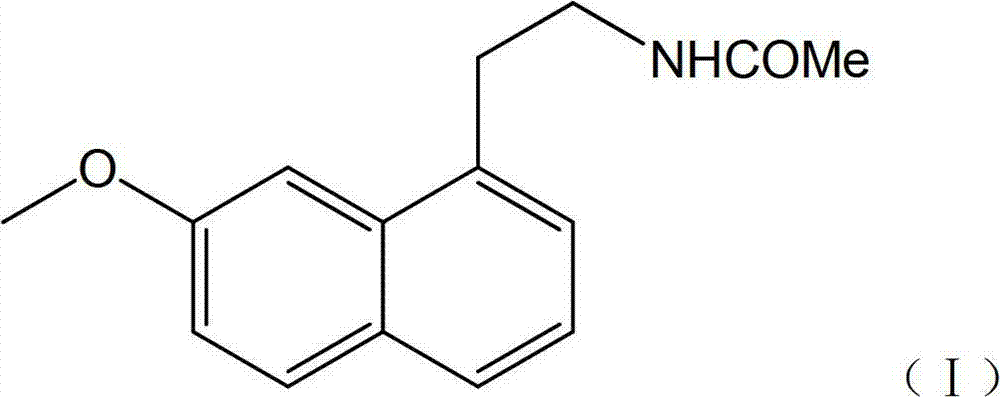
Novel method for preparing agomelatine
A methoxy, step 2 technology, applied in the preparation of carboxylic acid amides, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of complex route process, environmental pollution, many steps, etc., to reduce pollution, easy to use. Mild effect of operation and reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
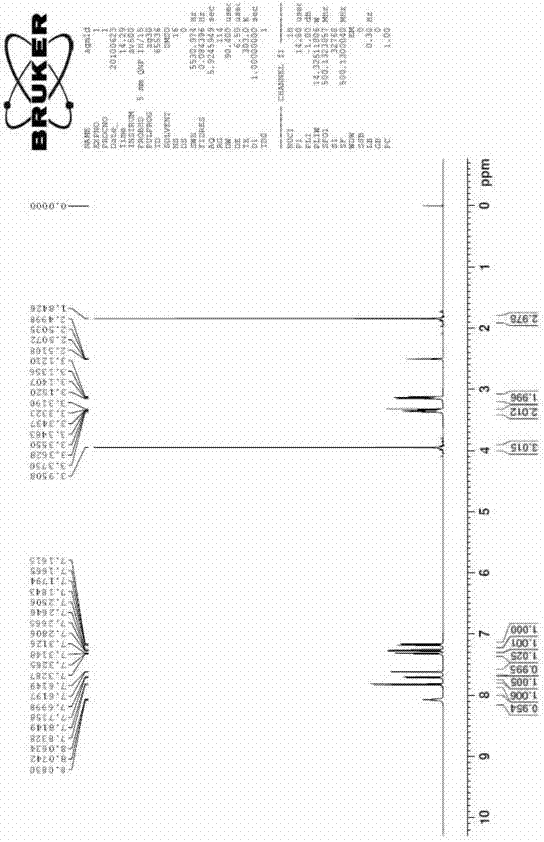
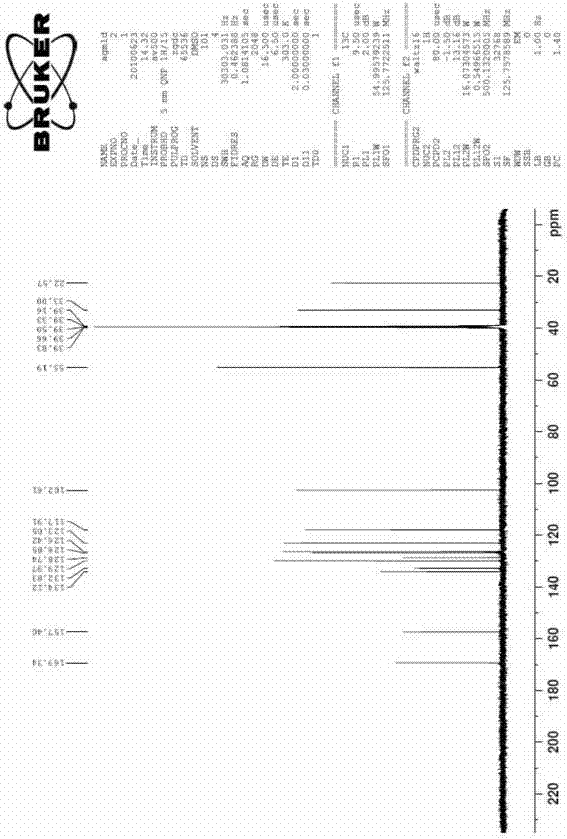
[0037] Take 10ml of concentrated sulfuric acid in a 50ml flask, slowly add 10g of 7-methoxy-1-naphthylacetonitrile under stirring, after stirring evenly, control the stirring reaction at 50°C for 2h, TLC tracking until there is no starting material in the reaction, the reaction is complete. After natural cooling, slowly pour it into 100ml of ice water, then adjust the pH to neutral and slightly alkaline with concentrated ammonia water, and precipitate a solid, filter, wash the filter cake with a little water, and dry to obtain 9.8g of white solid (7-methoxy -1-naphthyl)acetamide, yield 89.8%, melting point: 135-138°C.
[0038] Take the above (7-methoxy-1-naphthyl)acetamide solid 7.2g, sodium borohydride 3.2g, aluminum trichloride 15g, tetrahydrofuran 100ml into the reaction flask, reflux reaction until the substrate disappears. Add dilute hydrochloric acid, extract twice with ethyl acetate, adjust the aqueous layer to alkaline with ammonia water, then extract twice with ethyl ...
Embodiment 2
[0043] Take 15ml of concentrated sulfuric acid in a 50ml flask, slowly add 20g of 7-methoxy-1-naphthylacetonitrile under stirring, after stirring evenly, control the stirring reaction at 50°C for 2h, TLC tracking until there is no starting material in the reaction, the reaction is complete. After cooling down naturally, slowly pour it into 200ml of ice water, then adjust the pH to neutral and slightly alkaline with concentrated ammonia water, and precipitate a solid, filter, wash the filter cake with a little water, and dry to obtain 20g of white solid (7-methoxy- 1-naphthyl)acetamide, yield 91.6%, melting point: 135-138°C.
[0044] Take 18g of the above (7-methoxy-1-naphthyl)acetamide solid, 8g of sodium borohydride, 37g of aluminum trichloride, and 200ml of tetrahydrofuran into the reaction flask, and reflux until the substrate disappears. Add dilute hydrochloric acid, extract twice with ethyl acetate, adjust the aqueous layer to alkaline with ammonia water, then extract twi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com