1,1-Difluoroethylene-based copolymer, and use of the copolymer
A kind of copolymer, vinyl difluoride technology, applied in 1 field, can solve problems such as insufficient adhesion, achieve excellent adhesion and excellent peel strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
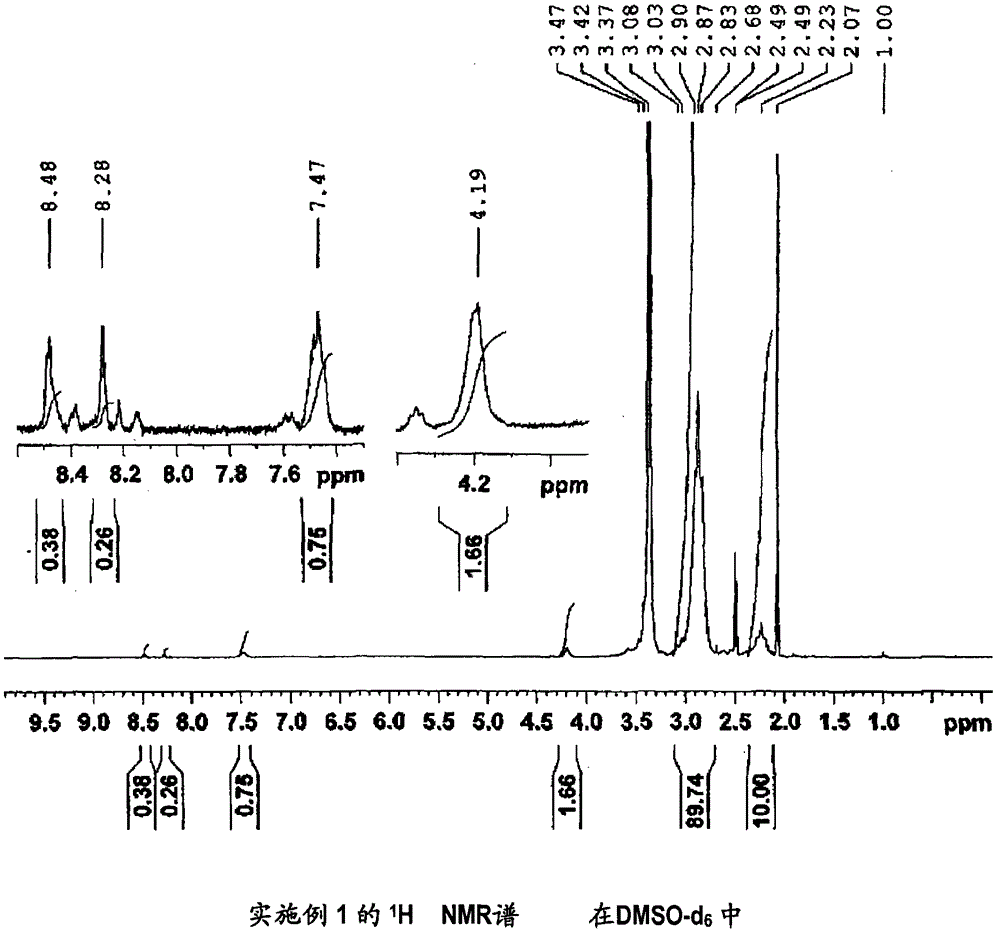
[0152] (Manufacture of 1,1-difluoroethylene-based copolymer (1))
[0153] In the 500ml three-neck flask with Dimrot cooling tube, dropping funnel and magnetic stirrer, add the carboxyl-containing 1,1-difluoroethylene copolymer (X1) made in 10.1g comparative example 1 and 150ml of N-methylpyrrolidone (NMP) and dissolved.
[0154] At room temperature, 5.30 g (0.0445 mol) of thionyl dichloride was dripped into the obtained solution, and after completion|finish of dripping, it heated and stirred at 80 degreeC for 1.5 hours.
[0155] After completion of heating and stirring, let cool to room temperature, add 4.34 g (0.0456 mol) of 3-hydroxypyridine, and further heat and stir at 90 for 1.5 hours.
[0156] After heating and stirring, the polymer was recovered by reprecipitation using a water / methanol mixed solvent as a poor solvent.
[0157] The inherent viscosity of the obtained vinylidene fluoride copolymer (1) was 2.07 dl / g.
[0158] In addition, measured under the same conditi...
Embodiment 2
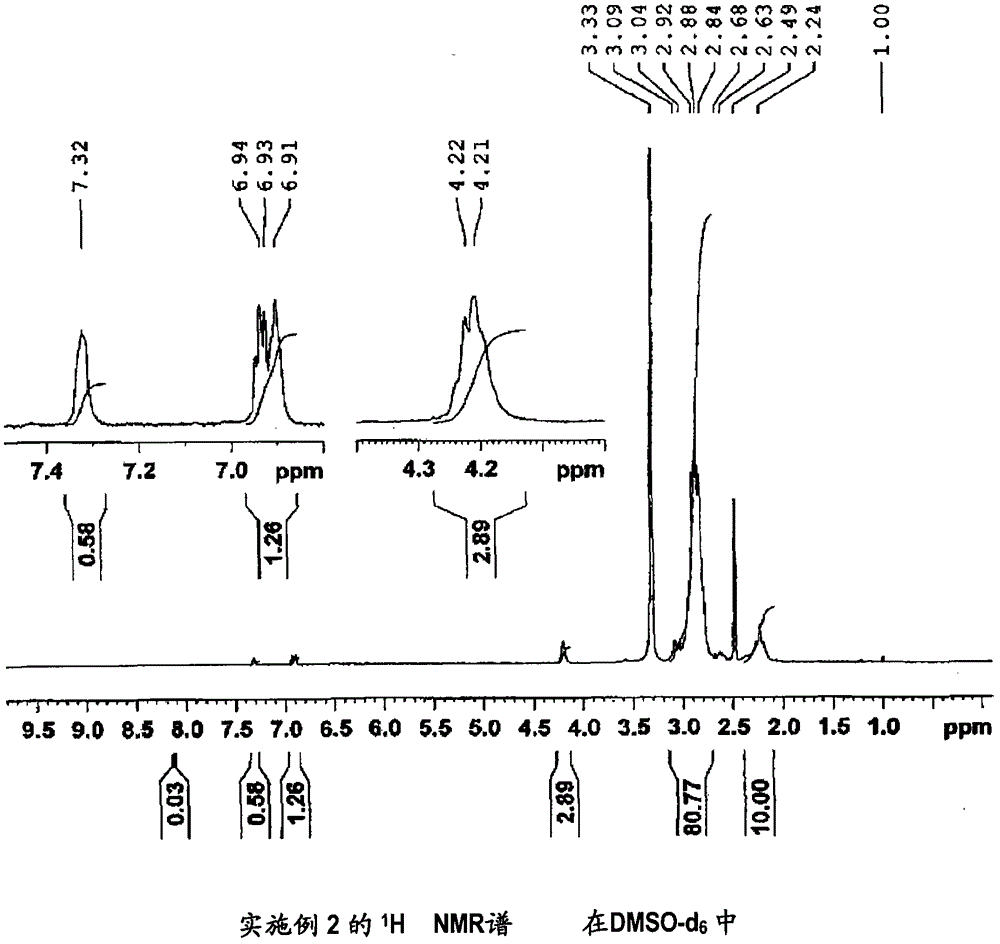
[0163] (Manufacture of 1,1-difluoroethylene-based copolymer (2))
[0164] It was synthesized in the same manner as in Example 1, except that 3-hydroxypyridine in Example 1 was changed to 2-(2-thienyl)ethanol.
[0165] The inherent viscosity of the obtained vinylidene fluoride copolymer (2) was 2.11 dl / g.
[0166] In addition, measured under the same conditions as Comparative Example 1 1 It was confirmed by H NMR spectrum that 74 mol% of the carboxyl groups in the carboxyl group-containing vinylidene fluoride copolymer (X1) produced in Comparative Example 1 was esterified. It should be noted that the 1,1-difluoroethylene-based copolymer (2) 1 H NMR spectrum is shown in figure 2 . Confirmation of the above-mentioned esterification rate is based on the integrated intensity of 1.84 of the peak derived from the three hydrogen atoms of the thiophene ring observed around 6.5 to 7.5 ppm and the peak derived from -C(O)O-CH observed around 4.2 ppm. 2 - The integral intensity of th...
Embodiment 3
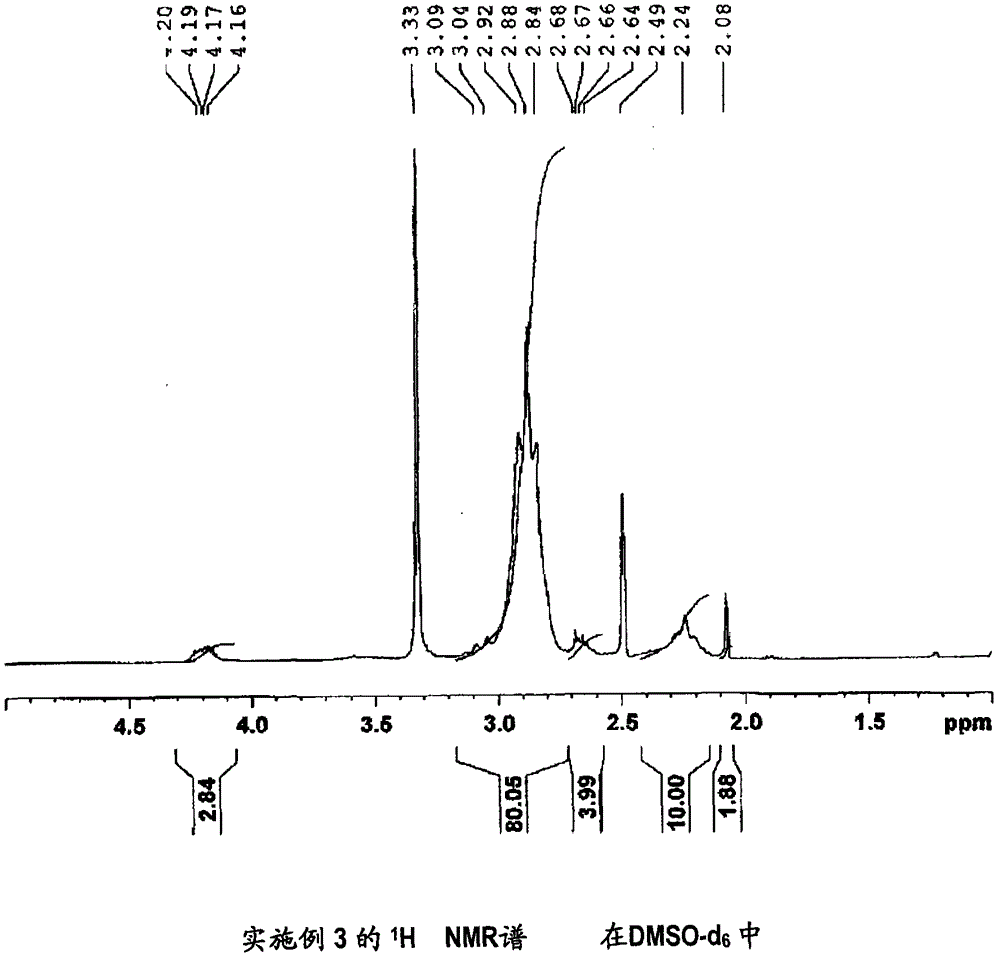
[0171] (Manufacture of 1,1-difluoroethylene-based copolymer (3))
[0172] It was synthesized in the same manner as in Example 1, except that 3-hydroxypyridine in Example 1 was changed to 2-(methylthio)ethanol.
[0173] The inherent viscosity of the obtained vinylidene fluoride copolymer (3) was 2.04 dl / g.
[0174] In addition, measured under the same conditions as Comparative Example 1 1 It was confirmed by H NMR spectrum that 79 mol% of the carboxyl groups in the carboxyl group-containing vinylidene fluoride copolymer (X1) prepared in Comparative Example 1 were esterified. It should be noted that the 1,1-difluoroethylene-based copolymer (3) 1 H NMR spectrum is shown in image 3 . Confirmation of the above-mentioned esterification rate was obtained from -S-CH observed around 2.1ppm 3 The integrated intensity of the peak of the methyl group of the structure is 1.88, and the -C(O)O-CH observed around 4.2ppm 2 - The integral intensity of the methylene peak of the structure ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com