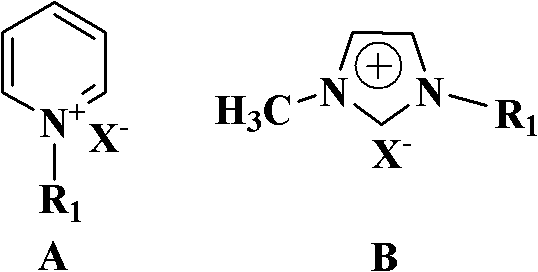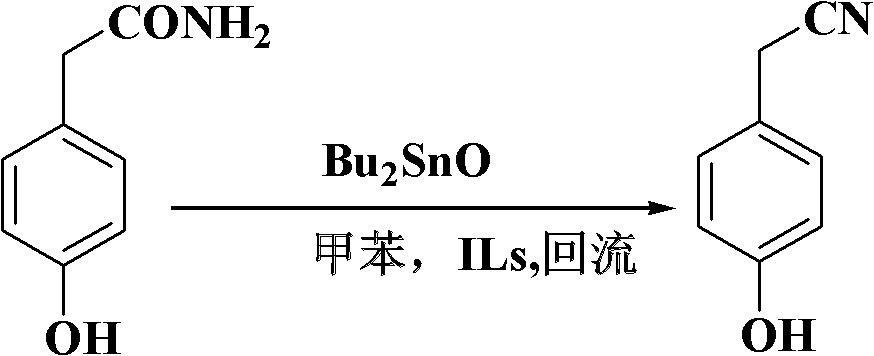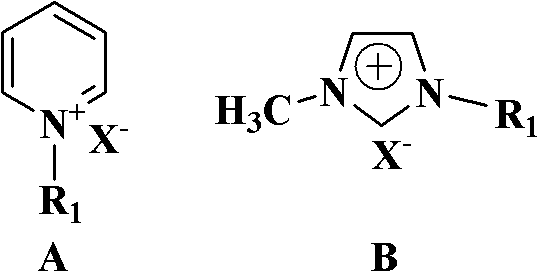Preparation method of hydroxybenzyl cyanide
A technology of hydroxyphenylacetonitrile and hydroxyphenylacetamide, which is applied in the field of preparation of p-hydroxyphenylacetonitrile, can solve the problems of high waste treatment cost, serious equipment corrosion, complicated treatment, etc., and achieve the advantages of simple operation, short route and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 5 grams (0.033mol) of p-hydroxyphenylacetamide, 0.41 grams (1.65mmol) of dibutyltin oxide, 0.10 grams of ionic liquid N-picoline bromide and 50 mL of dry toluene in a 100 mL single-necked bottle, and the reaction mixture The reaction was stirred at reflux. Reaction adopts TLC detection reaction to carry out degree, developing agent sherwood oil / ethyl acetate (volume ratio 2: 1), the R of p-hydroxyphenylacetonitrile f About = 0.6. After 19 hours of reaction, the reaction was completed, and the dibutyltin oxide and ionic liquid were removed by filtration while it was hot (which can be recycled and reused). After suction filtration and drying, 4.10 g of p-hydroxybenzonitrile was obtained with a yield of 94.2% and a purity of 98.7% by gas phase analysis.
[0024] m.p.: 69-71°C;
[0025] 1 HNMR (CDCl 3 , 400M), TMS): δ3.63 (s, 2H), 6.51 (s, br, 1H), 6.85-6.87 (d, 2H), 7.15-7.17 (d, 2H).
[0026] 13 CNMR (100M, CDCl 3 ,) δ 155.8, 129.3, 121.3, 118.6, 116.2, 22.8. ...
Embodiment 2
[0028] Add 10 grams (0.066mol) of p-hydroxyphenylacetamide, 0.82 grams (3.3mmol) of dibutyltin oxide, 0.20 grams of ionic liquid N-butylpyridine bromide and 120 mL of dry toluene in a 250 mL single-necked bottle, and the reaction mixture The reaction was stirred at reflux. The progress of the reaction was detected by TLC. After 19 hours of reaction, the reaction was completed, and the dibutyltin oxide and ionic liquid were removed by filtration while it was hot (which can be recycled and reused). After suction filtration and drying, 8.60 g of p-hydroxybenzonitrile was obtained with a yield of 97.7% and a purity of 98.8% by gas phase analysis.
Embodiment 3
[0030] Add 10 grams (0.066mol) of p-hydroxyphenylacetamide, 0.82 grams (3.3 mmol) of dibutyltin oxide, 0.20 grams of 1-methyl-3-ethylimidazolium bromide and 120 mL of dry toluene into a 250 mL single-necked bottle , the reaction mixture was stirred under reflux. The progress of the reaction was detected by TLC. After 18 hours of reaction, the reaction was completed, and the dibutyltin oxide and the ionic liquid were removed by filtration while hot (which can be recycled and reused). After suction filtration and drying, 8.50 g of p-hydroxybenzonitrile was obtained with a yield of 96.6% and a purity of 98.8% by gas phase analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com