Composition containing candesartan and amlodipine, preparation process, testing process and application thereof
A kind of technology of amlodipine and candesartan, applied in the field of compositions, can solve problems such as high cost, unsatisfactory product index, unstable quality and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The sample preparation and inspection of embodiment 1, composition powder
[0072] Preparation: Take 6g of candesartan cilexetil, 7g of amlodipine, 80g of mannitol, 6g of croscarmellose calcium, and 6g of cornstarch, mix evenly, make a powder, and pack it into 1000 bags.
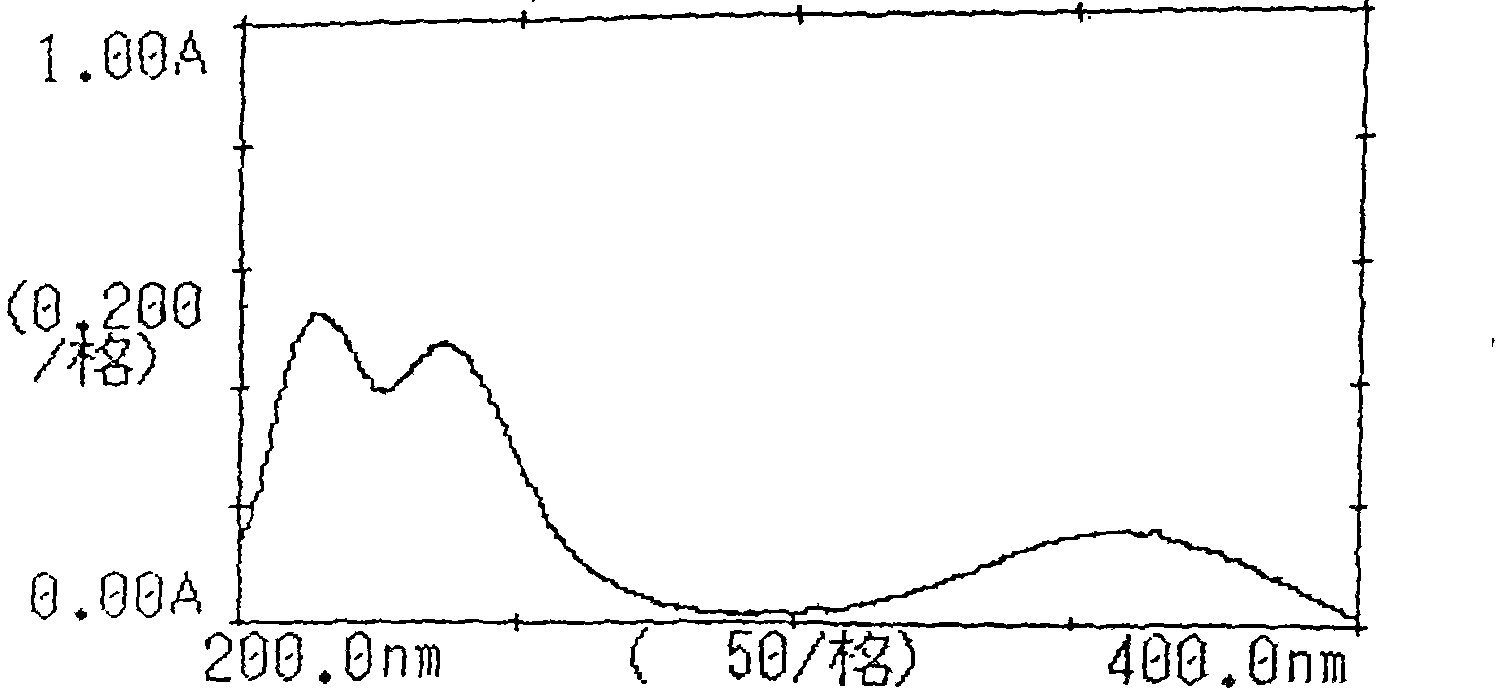
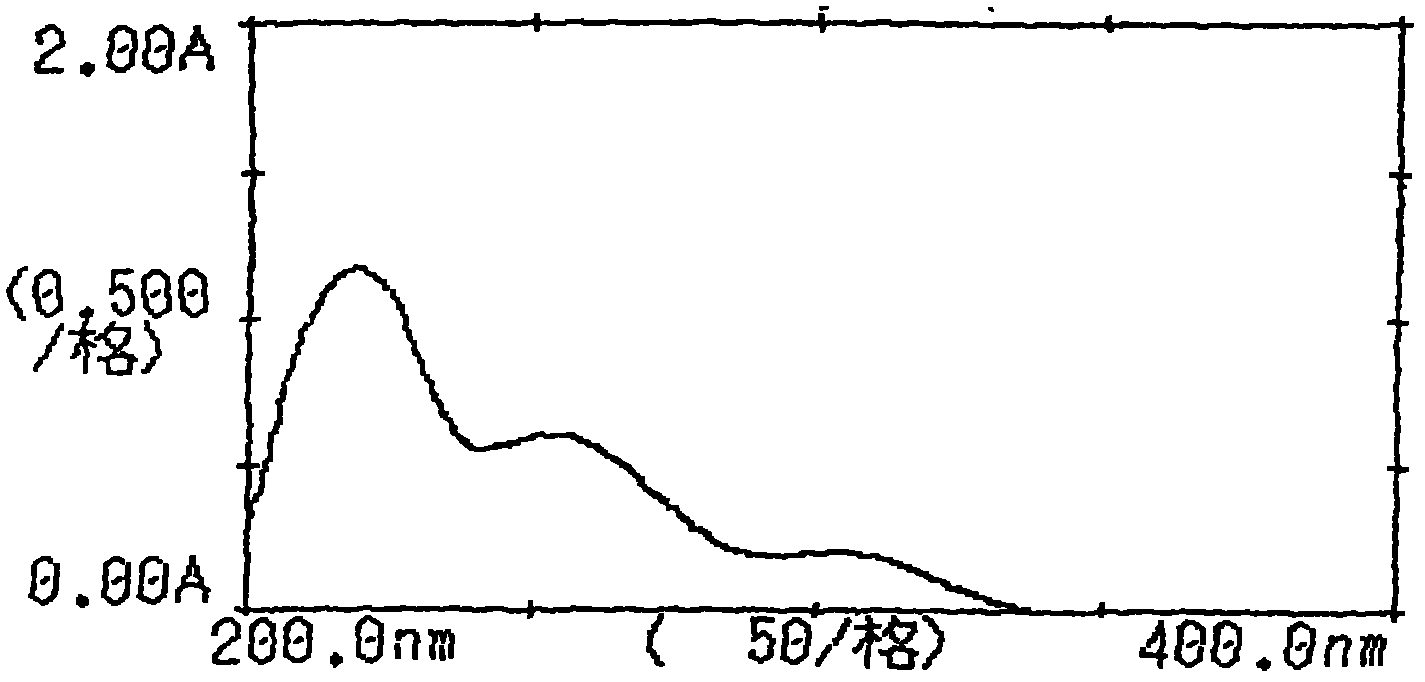
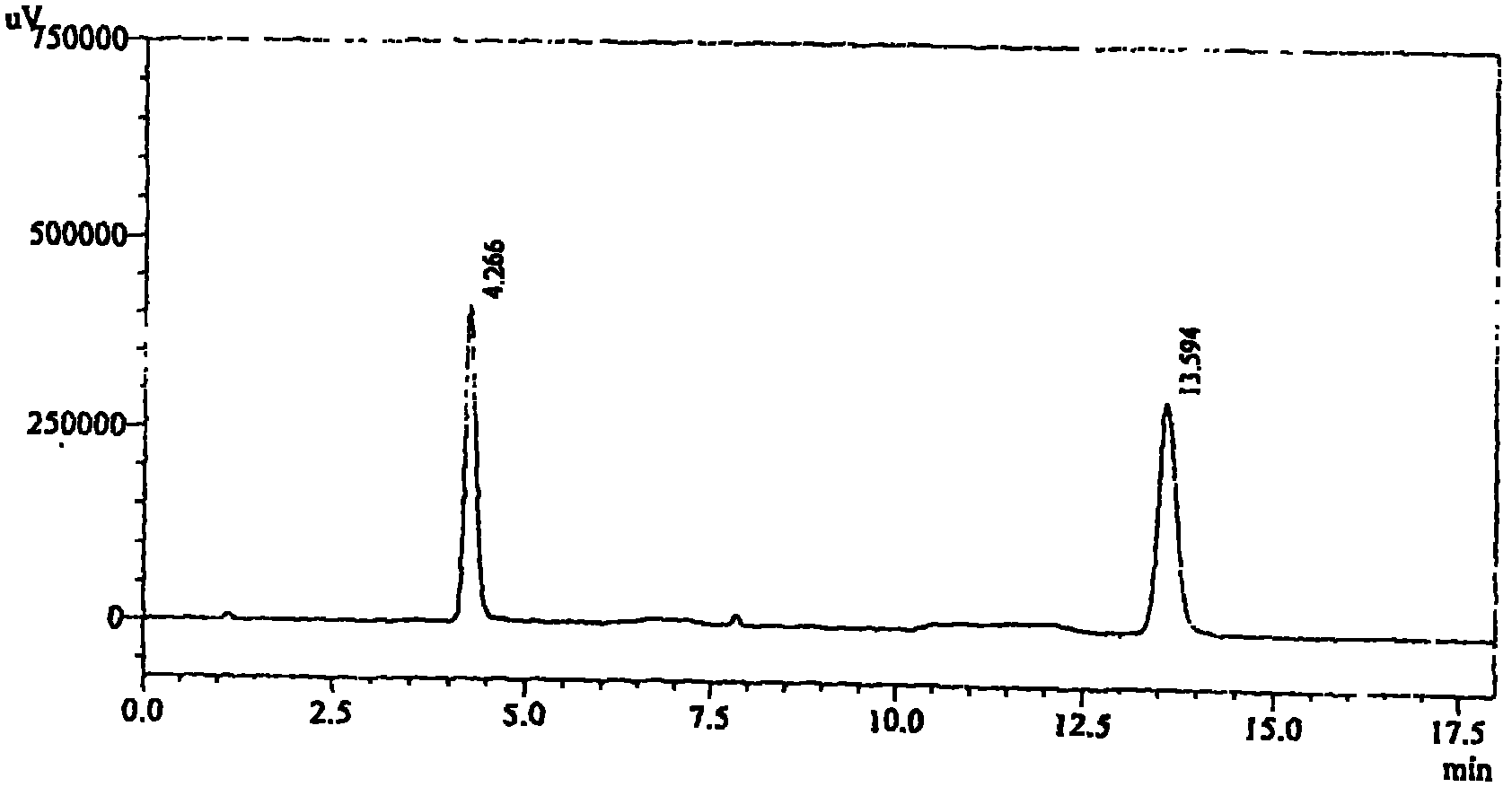
[0073] Inspection: 【Inspection】Related substances of candesartan cilexetil take this product, accurately weigh an appropriate amount of fine powder (approximately equivalent to 18 mg of candesartan cilexetil), put it in a 50ml measuring bottle, add acetonitrile-water (2:3) for ultrasonic treatment Dissolve and dilute to the mark, filter, take the subsequent filtrate as the test solution; accurately weigh about 22 mg of the candesartan cilexetil reference substance and put it in a 50ml measuring bottle, add acetonitrile-water (2:3) to dissolve and dilute to the mark , shake well, filter, accurately measure 1ml, put it in a 100ml measuring bottle, add acetonitrile-water (2:3) to dilute to the mark, shak...
Embodiment 2
[0082] Embodiment 2, preparation and inspection of composition capsule
[0083]Preparation: get 10kg candesartan, 2.5kg amlodipine, 100kg mannitol, 5kg croscarmellose sodium, 35kg microcrystalline cellulose and mix evenly, mix with 2% hypromellose ethanol (ethanol concentration 30%) solution about 20L to make soft materials, granulate with a 20-mesh sieve with an aperture, dry at 58°C, granulate with a 16-mesh sieve with an aperture, mix the granules with 0.5kg magnesium stearate evenly, and pack 1,000,000 capsules Grains, that is.
[0084] Inspection: [Inspection] Candesartan-related substances take 10 capsules of this product, accurately weigh the contents, grind finely, and accurately weigh an appropriate amount of fine powder (approximately equivalent to 22mg of candesartan), put it in a 50ml measuring bottle and add acetonitrile -Water (4: 1) sonication makes dissolving and dilutes to the scale, filters, and gets the subsequent filtrate as the test solution; accurately w...
Embodiment 3
[0093] Embodiment 3, preparation and inspection of composition granules
[0094] Preparation: Mix 8kg of candesartan cilexetil, 2.5kg of amlodipine besylate, 95kg of mannitol, 3kg of croscarmellose sodium, 18kg of microcrystalline cellulose and an appropriate amount of starch as a base material, and mix them with an appropriate amount of water One-step granulation for the wetting agent, divided into 500,000 bags, 1g per bag, to obtain.
[0095] Inspection: [Inspection] Take 10 bags of related substances of candesartan cilexetil, accurately weigh them, grind them finely, accurately weigh an appropriate amount of fine powder (approximately equivalent to 20 mg of candesartan cilexetil), put it in a 50ml measuring bottle and add acetonitrile -Water (3: 2) sonication makes dissolving and dilutes to scale, filters, and gets the subsequent filtrate as the test solution; Accurately weighs 20 mg of candesartan cilexetil reference substance and puts it in a 50ml measuring bottle, adds a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com