Preparation method of tetracyclic terpene compound
A compound, C1-C12 technology, applied in the field of preparation of tetracyclic terpenoids, can solve the problems of ecological damage, low yield, single product, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
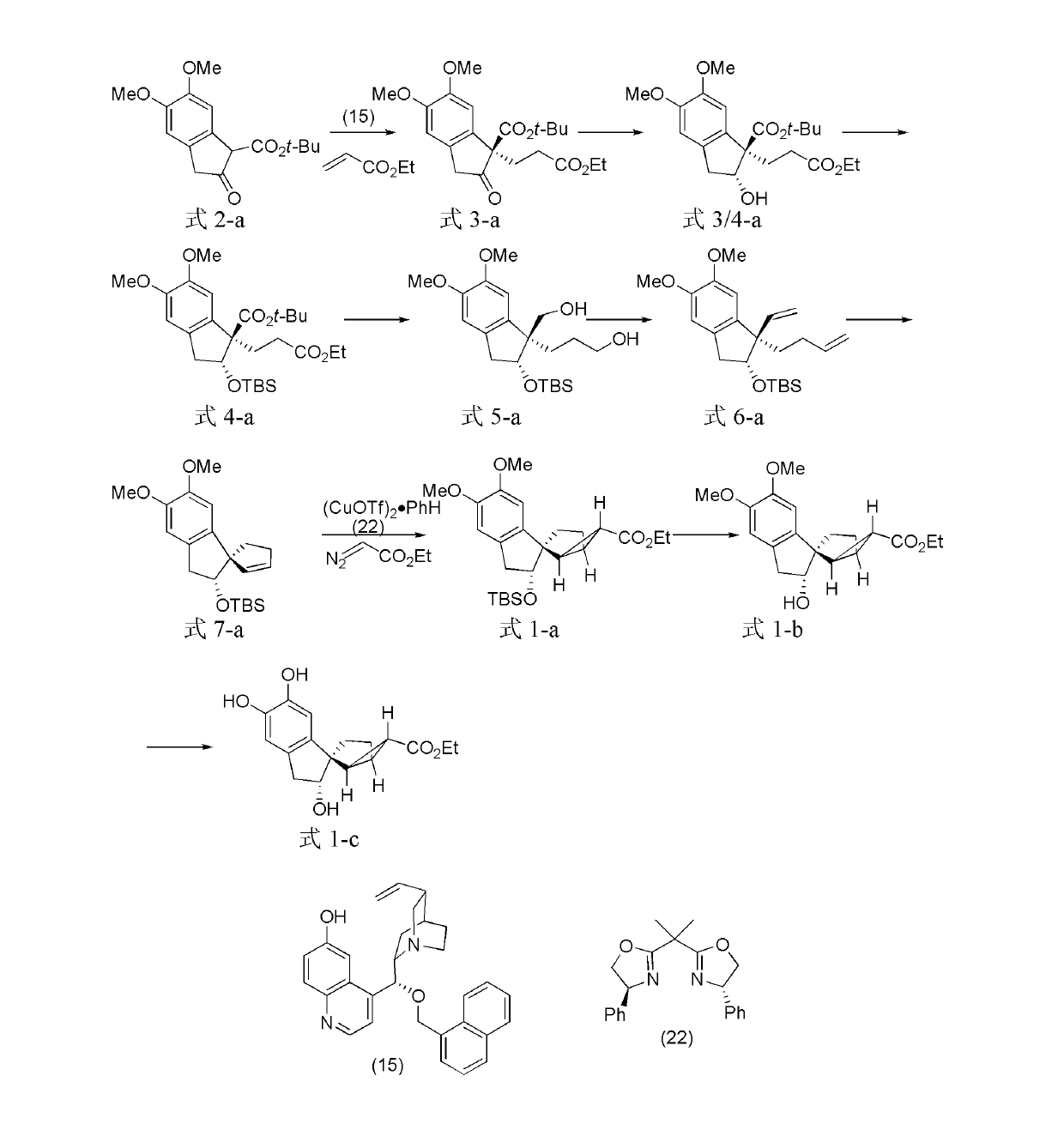
[0082] The preparation of embodiment 1, tetracyclic terpenoids
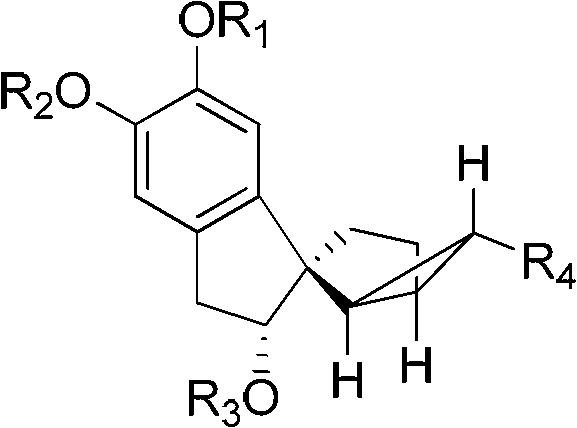
[0083] The structural formulas of the prepared tetracyclic terpenoids are shown in formula 1-a, formula 1-b, and formula 1-c.
[0084]
[0085] Formula 2-a Formula 3-a Formula 3 / 4-a
[0086]
[0087] Formula 4-a Formula 5-a Formula 6-a
[0088]
[0089] Formula 7-a Formula 1-a Formula 1-b
[0090]
[0091] Formula 1-c
[0092]
[0093] In the above formula, "TBS" represents "tert-butyldimethylsilyl".
[0094] The compound shown in 5.85g formula (2-a) and 6.01g ethyl acrylate are dissolved in 40mL chloroform, add the compound shown in 0.90g formula (15) (compound shown in formula (2-a), ethyl acrylate and formula The molar fraction ratio of the compound shown in (15) is 1: 3: 0.1), reacted for 60 hours at 25° C., concentrated, and crossed a flash chromatographic column to obtain the compound shown in 5.65g formula (3-a) (72%, 90% ee ) (analytical conditions: high performance liquid chromatograp...
Embodiment 2
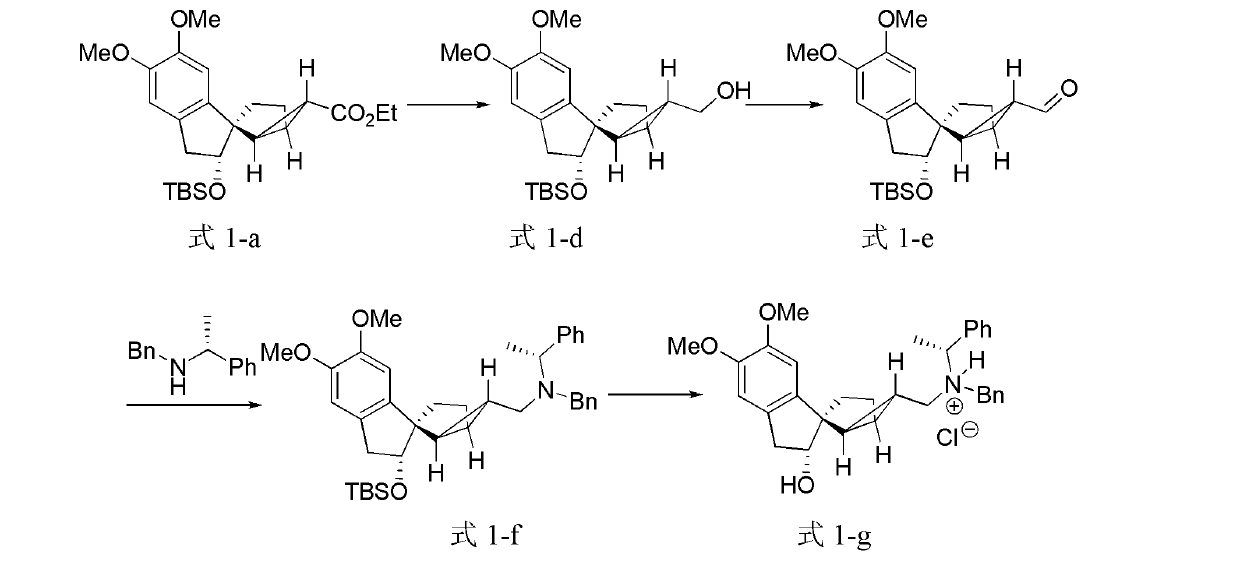
[0106] Embodiment 2, the preparation of tetracyclic terpenoids
[0107] The structural formulas of the prepared tetracyclic terpenoids are shown in formula 1-d, formula 1-e, formula 1-f, and formula 1-g.
[0108]
[0109] Formula 1-a Formula 1-d Formula 1-e
[0110]
[0111] Formula 1-f Formula 1-g
[0112] Under nitrogen, dissolve 0.495g of the compound represented by formula (1-a) in 11mL of dichloromethane, place it in a -78°C reaction bath, add 2.8mL of diisobutylaluminum hydride (1M n-hexane solution) , moved to an ice-water bath to react for 1 hour. Add 0.5 mL of methanol to quench the reaction, add 20 mL of saturated sodium potassium tartrate solution, stir at room temperature for 0.5 hours, extract with dichloromethane (10 mL x 3), combine the organic phases, dry over anhydrous magnesium sulfate, filter, concentrate, and pass through a flash chromatography column Purification gave 0.346 g of the compound represented by formula (1-d) (78%) as a colorless oil. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com