Enrofloxacin slow-release micropill for livestocks, and preparation method of same
A technology of enrofloxacin and sustained-release pellets, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, which can solve the problem of limiting the application field of enrofloxacin and affecting convenience And economy, enrofloxacin taste bitter and other issues, to achieve the effect of improving the color change when exposed to light, reducing the number of medications, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
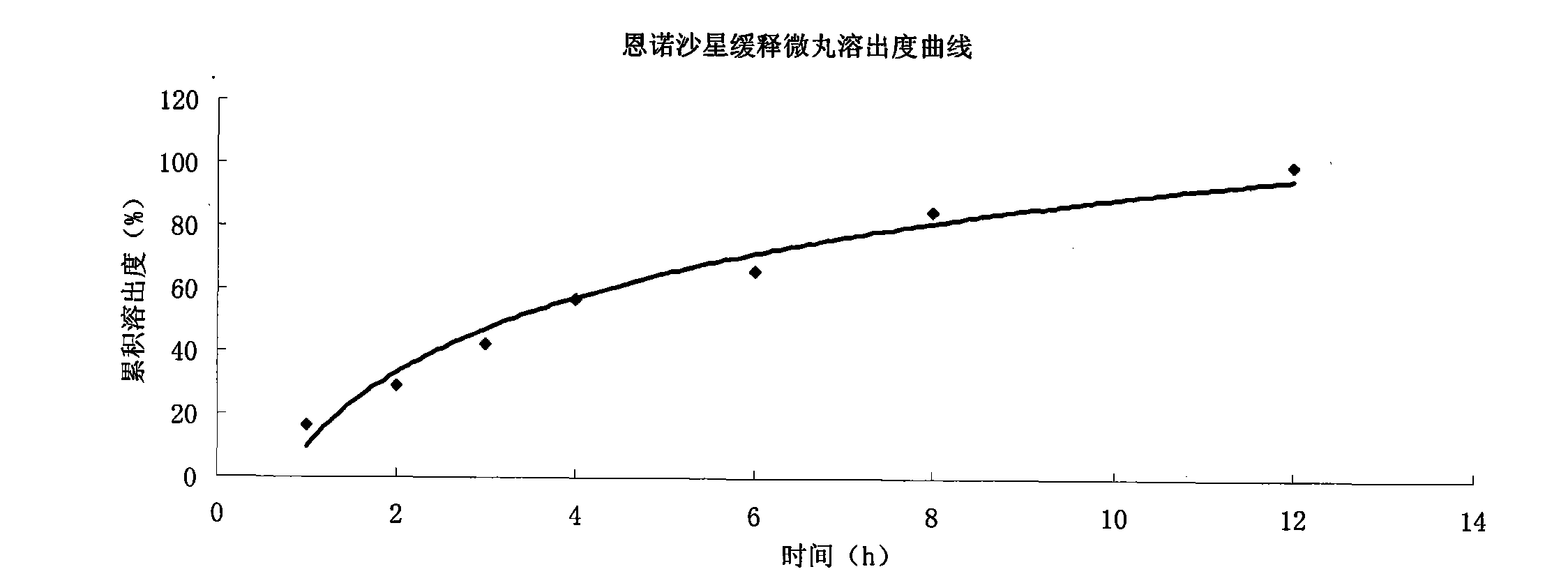
Image
Examples
Embodiment 1
[0021] The preparation of embodiment 1 enrofloxacin sustained-release pellets
[0022] Raw materials: enrofloxacin, microcrystalline cellulose, corn starch, medicinal dextrin, sodium carboxymethyl starch, sodium carboxymethyl cellulose, ethyl cellulose, talcum powder.
[0023] Preparation process: Mix the auxiliary materials microcrystalline cellulose, corn starch, medicinal dextrin and sodium carboxymethyl starch in a weight ratio of 1:1:1:2, and fully mix with enrofloxacin, add 0.3% carboxymethyl starch Sodium methyl cellulose is a soft material made of binder. The soft material is placed in an extrusion-spheronizer, the extrusion speed is set at 15r / min, and the spheronization speed is 100r / min to make a pellet core.
[0024] Place the pellet core in a fluidized bed to dry, and coat the surface of the pellet with an ethyl cellulose film coating until the weight gain is 6%. The parameters are: the fluidized bed is preheated to 40°C, the coating temperature is 30°C, atomized ...
Embodiment 2
[0026] Ingredients: Enrofloxacin, Microcrystalline Cellulose, Corn Starch, Crospovidone, Sodium Carboxymethyl Cellulose, Cellulose Acetate, Titanium Dioxide.
[0027] Preparation process: Mix the auxiliary materials microcrystalline cellulose, corn starch, and crospovidone according to the weight ratio of 1:1:2, and fully mix them with enrofloxacin, and use 0.5% sodium carboxymethylcellulose as the viscosity The soft material is prepared from the mixture, the soft material is placed in the extrusion-spheronizer, the extrusion speed is set to 20r / min, and the spheronization speed is 200r / min to make the pellet core.
[0028] Put the pellet core in a fluidized bed to dry, and coat the surface of the pellet with a cellulose acetate film coating until the weight gain is 8%. The parameters are: fluidized bed preheated to 45°C, coating temperature 40°C, atomization pressure 0.6MPa, spray liquid flow rate 30ml / min, after spraying, continue to dry for 30min, then dry and sieve, take 2...
Embodiment 3
[0030] Raw materials: enrofloxacin, microcrystalline cellulose, lactose, sodium starch glycolate, maltodextrin, starch, acrylic resin, talc.
[0031] Preparation process: Mix the auxiliary materials microcrystalline cellulose, lactose, maltodextrin and sodium carboxymethyl starch in a weight ratio of 2:1:2:2, and fully mix with enrofloxacin, and use 8% starch slurry as Adhesives are used to make soft materials, the soft materials are placed in the extrusion-spheronizer, the extrusion speed is set to 25r / min, and the spheronization speed is 500r / min to make pellet cores.
[0032] Place the pellet core in a fluidized bed to dry, and coat the surface of the pellet with a polyacrylic resin film coating until the weight gain is 10%. The parameters are: fluidized bed preheated to 50°C, coating temperature 45°C, atomization pressure 0.7MPa, spray liquid flow rate 50ml / min, after spraying, continue to dry for 30min, then dry and sieve, take 20-40 mesh pellets, namely enrofloxacin sust...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com