Recombinant adeno-associated virus increasing targeted transduction efficiency of adeno-associated virus, and application of recombinant adeno-associated virus
A virus and transduction technology, applied to recombinant adeno-associated virus and its application field, can solve the problems of low infection efficiency, poor local action specificity, difficult to achieve therapeutic effect, etc., and achieves increased transduction efficiency, increased targeting, The effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
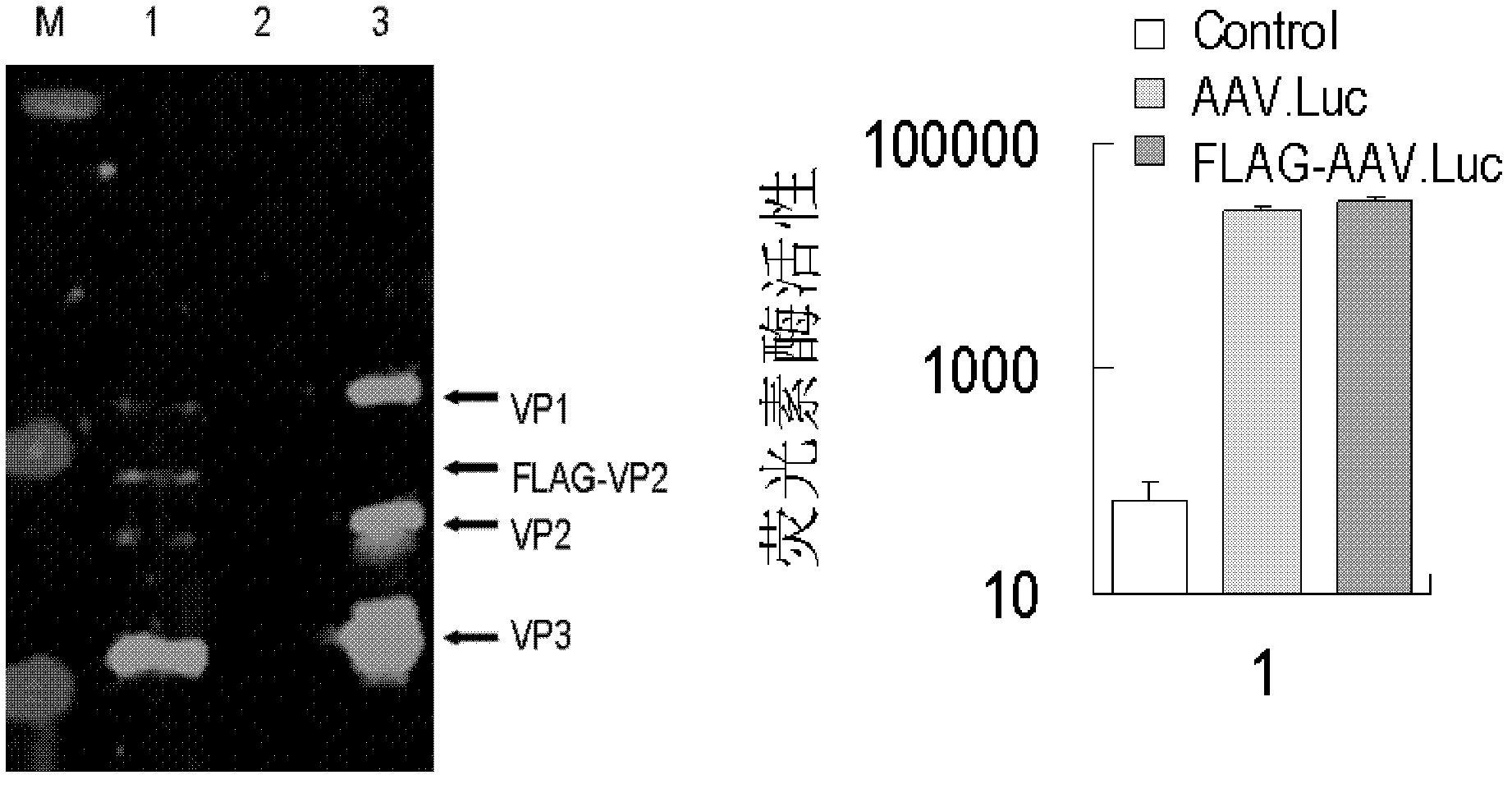
[0151] Construction and identification of embodiment recombinant four-plasmid adeno-associated virus (FLAG-AAV.Luc)
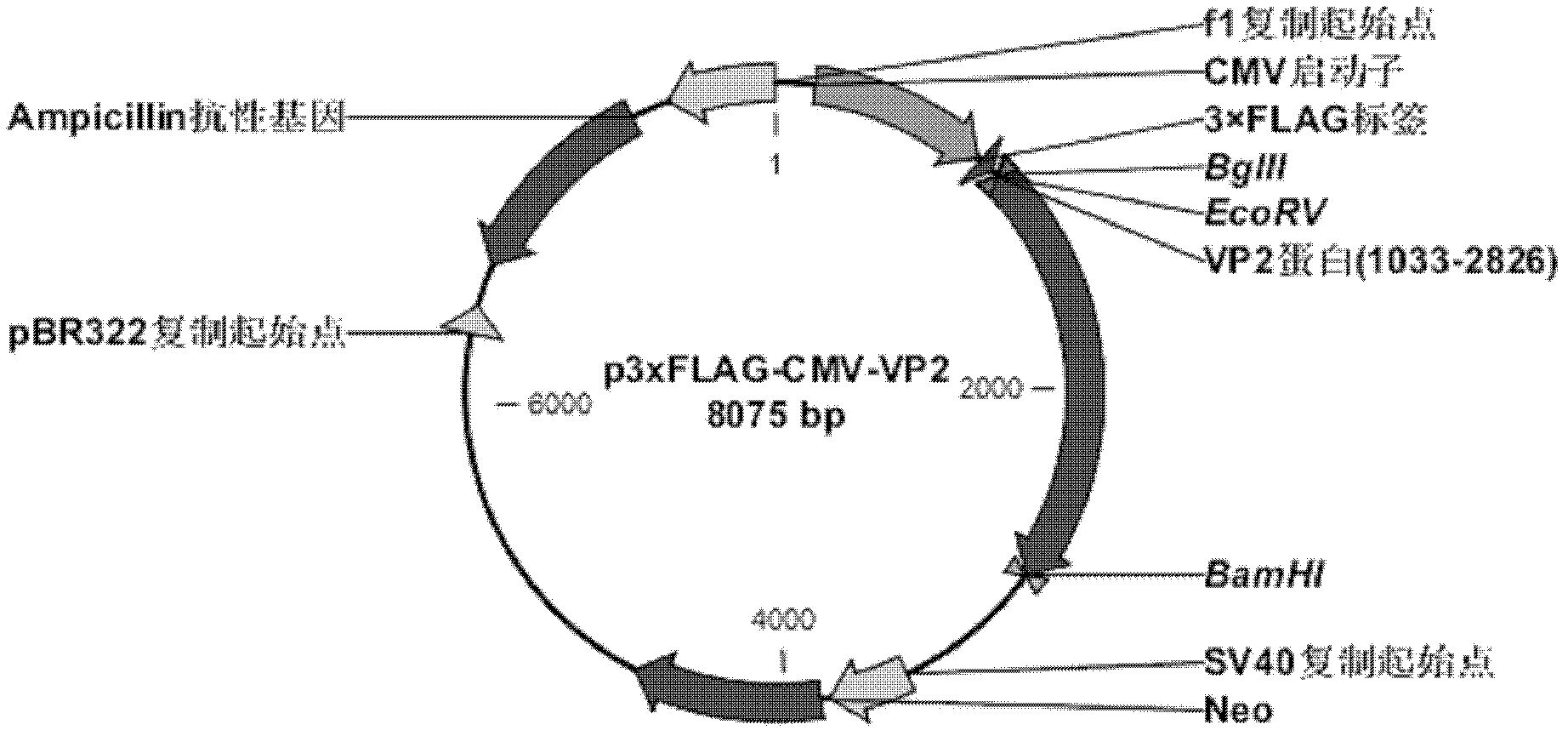
[0152] 1. Construction and identification of recombinant expression plasmid p3×FLAG-CMV-VP2
[0153] 1.1 Using pAAV-RC as a template, amplify the VP2 sequence by PCR, and the primers are:
[0154] Table 1 Primer Sequence
[0155]
[0156] The two parts in italics are the artificially added EcoR V and BamH I restriction sites respectively.
[0157] 1.2 Use EcoR V and BamH I to cut the empty vector p3×FLAG-CMV-10 and the VP2 sequence amplified by PCR respectively, and connect the VP2 sequence to the p3×FLAG-CMV-10 vector in the correct reading frame to obtain the expression of FLAG Plasmid p3xFLAG-CMV-VP2 fusion protein with VP2.
[0158] 1.3 Identification of the obtained recombinant plasmid vector.
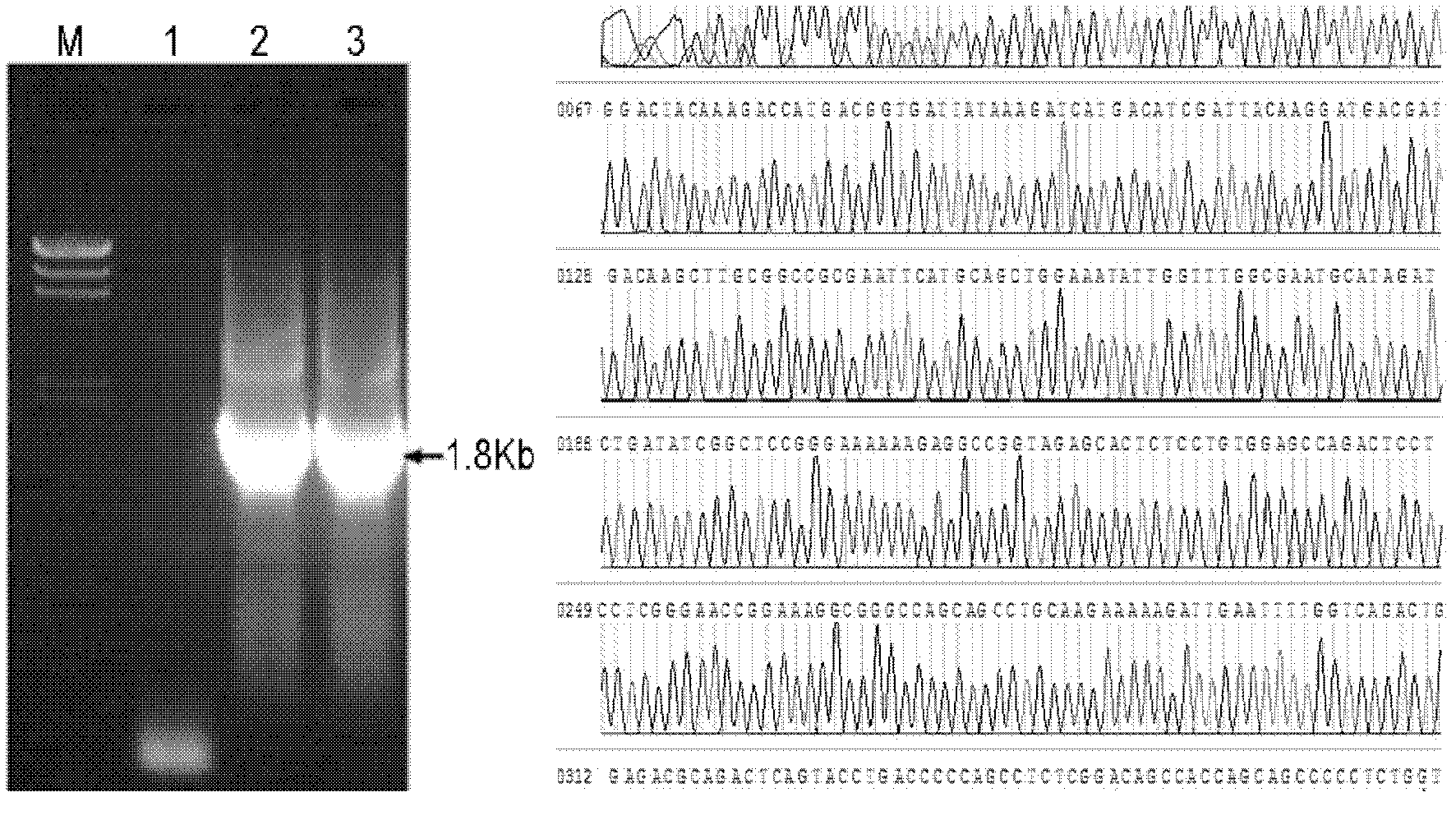
[0159] Obtain the VP2 sequence of AAV virus by PCR amplification ( figure 1 ), figure 1 The middle arrow points to the target band of the corresponding se...
experiment example 2
[0206] Experimental example 2 Application of the four-plasmid recombinant virus with FLAG tag of the present invention in absorbable materials
[0207] 1.PLGA
[0208] experimental method:
[0209] Poly(lactic-co-glycolic acid) (poly(lactic-co-glycolic acid), PLGA) (purchased from Shandong Medical Instrument Factory) was used as solid phase material, coated with 1 mg / ml collagen activated by EDAC, and dried at room temperature. 20mM SPDP was incubated at room temperature for 4 hours, and antibody immobilization was performed after washing with PBS. Use non-specific antibody IgG (50mg / ml) as negative control group, Anti-FLAG monoclonal antibody (50mg / ml) as positive control group, and incubate overnight at 4°C. After washing with PBS, AAV.LacZ and FLAG-AAV.LacZ were incubated at room temperature for 2 hours. After washing with PBS, Hela cell suspension was added, after 2 hours of attachment, 10% FBS was added to incubate for 48 hours, stained with β-galactosidase in situ sta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com