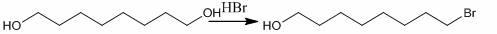Synthesis process of 10-HDA (10-hydroxy-2-decenoic acid)
A 10-HDA and 1.10-HDA technology, applied in the field of new synthesis technology, can solve the problems of low yield, high cost, harsh reaction conditions, etc., and achieve the effect of high yield, simple method and improved utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Synthesis of 8-bromo-1-octanol
[0019] Add 7.3 g ( 50 mmol) of 1,8-octanediol, 3.3 ml ( 80 mmol) of hydrobromic acid, 0.15 g of iodine (2% of the main material) and 150 ml of toluene to the reaction flask in sequence, and perform a water separation reaction under stirring After 10 h, toluene was recovered to obtain a yellow mixture. The mixture was separated by column chromatography (ф 8 × L 38 cm), eluted with chloroform:methanol (50:1), and evaporated to dryness in the eluted part of 0.5-1.0 L to obtain a light yellow oil 2, Total 9.62 g, yield 92.5%. Product detection: yellow oil; IRυ cm -1 : 3410 (OH), 2916, 2862 (C-H), 642 (C-Br); 1 H NMR (CDCL 3 ): δ: 3.53 ( t, 2 H, H-8 ), 3.35 (t, 2 H, H-1) , 1.75 (m, 2 H, H-7), 1.45 (m, 2 H, H-2 ), 1.34 (m, 2 H, H-3), 1.24 (m, 6 H, H-(4-6)); ESI-MS m / z 210 [M+H] + (positive mode).
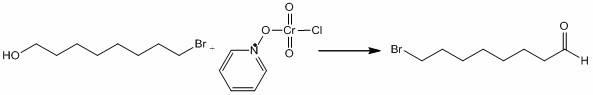
[0020] Synthesis of Bromooctylaldehyde
[0021] Add 6.46 g (30.1 mmol) of 8-bromo-1-octanol and 60 ml of dichloromethane into the r...
Embodiment 2
[0025] Synthesis of 8-bromo-1-octanol
[0026] Add 3.85 g (25 mmol) of 1,8-octanediol, 1.5 ml (35 mmol) of hydrobromic acid, 0.07 g of iodine (2% of the main material) and 75 ml of toluene to the reaction flask in sequence, and perform water separation reaction under stirring After 10 h, toluene was recovered to obtain a yellow mixture. The mixture was separated by column chromatography (ф 8 × L 38 cm), eluted with chloroform:methanol (50:1), and evaporated to dryness in the eluted part of 0.5-1.0 L to obtain a light yellow oil 2, Total 2.40 g, yield 92.3%.
[0027] Synthesis of Bromooctylaldehyde
[0028] Add 2.40 g (11.2 mmol) of 1-bromooctanol and 25 ml of dichloromethane into the reaction flask, add 6.80 g (13 mmol) of PCC into the reaction flask, and react for 2 h while stirring, during which Keep the reaction temperature at 20-30°C. After the reaction is completed, use silica gel column chromatography and vacuum distillation to recover dichloromethane to obtain 1....
Embodiment 3
[0032] Synthesis of 8-bromo-1-octanol
[0033]Add 385 g (2.5 mol) of 1,8-octanediol, 170 ml (4 mol) of hydrobromic acid, 7 g of iodine (2% of the main material) and 7.5 L of toluene in sequence in the reaction flask, and stir for 10 h under water separation Afterwards, toluene was recovered to obtain a yellow mixture. The mixture was separated by column chromatography (ф 15 × L 3.8 m), eluted with chloroform:methanol (50:1), and evaporated to dryness in the eluted part of 0.5-10 L to obtain a light yellow oil 2, Total 240 g, yield 92.3%.
[0034] Synthesis of Bromooctylaldehyde
[0035] Add 240 g (1.12 mol) of 1-bromooctanol and 2.5L methylene chloride into the reaction flask, add 680 g (1.3 mol) of PCC into the reaction flask, and react for 2 h while stirring, during which Keep the reaction temperature at 20-30 °C. After the reaction is completed, use silica gel column chromatography and vacuum distillation to recover dichloromethane to obtain 198 g of yellow oil, with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com