Polylactide electrospinning composite film for in-situ gel encapsulating and preparation method thereof
A technology of polylactide and composite membrane, which is applied in spinning solution preparation, textile and papermaking, non-woven fabrics, etc., to achieve the effects of simple preparation process, controllable drug release rate, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
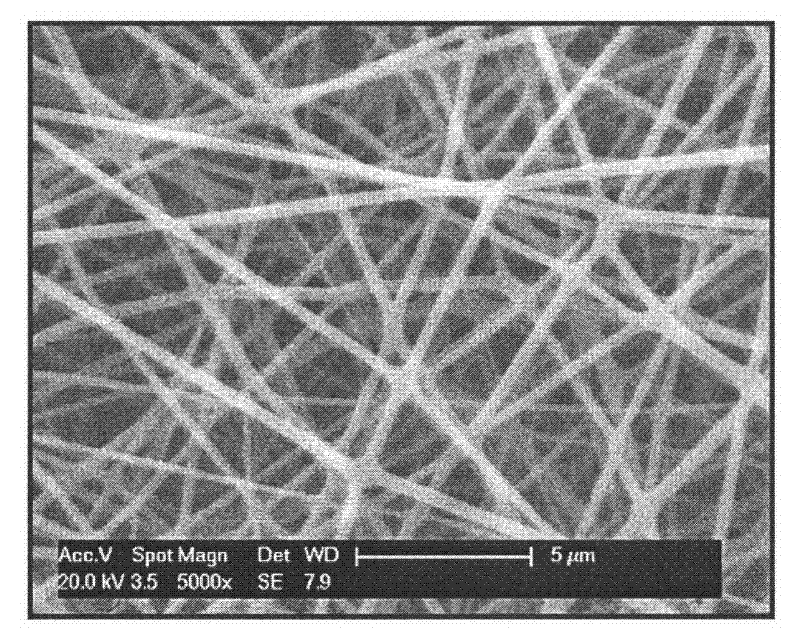
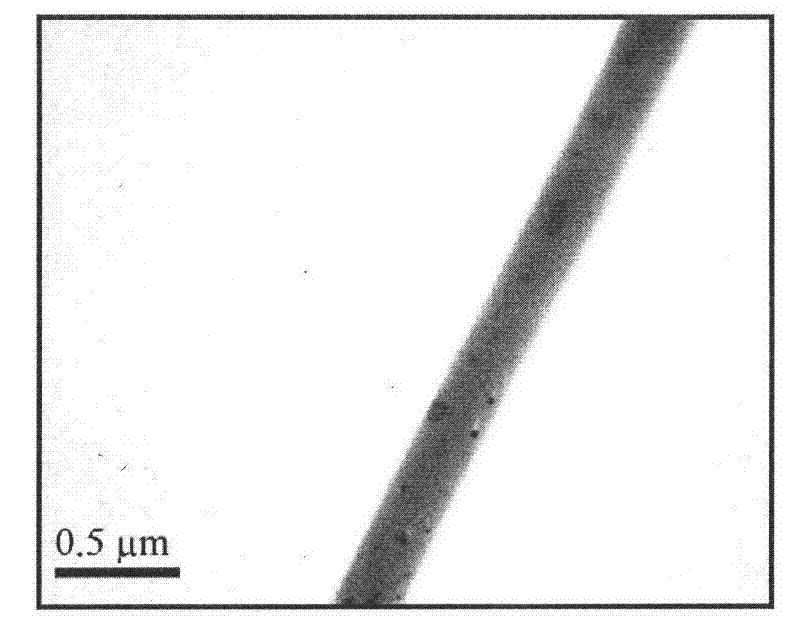
[0019] 6mg of CS-SH and 0.4 mg of PEGDA were dissolved in 1 mL of PBS (0.1 M, pH 7.4) as the aqueous phase. Then, 8 mg of F127 was dissolved in a mixed solvent of 0.8 mL of chloroform and 0.2 mL of DMF as an oil phase. Then add 120mg PLGA (LA:GA=3:1, ) is dissolved in the oil phase solution obtained above. Take 40 μL of the above-prepared water phase and disperse it in 1 mL of the above-prepared oil phase, stir (3000r / min) and mix for 5-30 minutes to form a W / O dispersion. The prepared W / O dispersion was electrospun using a single-needle electrospinning device with a voltage of 14kV, a flow rate of the dispersion of 0.4mL / h, and a receiving distance of 18cm for electrospinning. After 12 hours, the collected fibers were electrospun with a diameter of 200nm to 500nm. The composite membrane has a thickness of 40 μm to 70 μm, and the content of chitosan gel is 0.1% to 0.2%. The scanning electron micrographs of the prepared chitosan gel / PELCL electrospun composite membrane ar...
Embodiment 2
[0021] 40 mg of Gtn-SH and 2 mg of PEGDA were dissolved in PBS as the aqueous phase. Then, 5 mg of F127 was dissolved in a mixed solvent of 0.8 mL of chloroform and 0.2 mL of DMF as an oil phase. Further, 120 mg of PLGA was dissolved in the oil phase solution obtained above. Take 40 μL of the above-prepared water phase and disperse it in 1 mL of the above-prepared oil phase, stir (3000r / min) and mix for 5-30 minutes to form a W / O dispersion. The prepared W / O dispersion was electrospun using a single-needle electrospinning device with a voltage of 12kV, a flow rate of the dispersion of 0.3mL / h, and a receiving distance of 20cm for electrospinning. After 12 hours, the collected fibers were electrospun with a diameter of 200nm to 500nm. The composite film has a thickness of 40 μm to 70 μm, wherein the content of gelatin gel is 1% to 2%. Taking HRP as a drug model, the encapsulation rate is above 70% (mass), and the burst release rate is below 20%, which can achieve the purpose ...
Embodiment 3
[0023] 5mg CS-SH and 0.5 mg of PEGDA were dissolved in PBS as the aqueous phase. Then, 10 mg of Span 80 was dissolved in a mixed solvent of 0.9 mL of chloroform and 0.1 mL of DMF as an oil phase. Then 80mg PELCL (LA: CL = 3: 1, ) is dissolved in the oil phase solution obtained above. Take 67 μL of the above-prepared water phase and disperse it in 1 mL of the above-prepared oil phase, stir (3000r / min) and mix for 5-30 minutes to form a W / O dispersion. The prepared W / O dispersion was electrospun using a single-needle electrospinning device with a voltage of 14kV, a flow rate of the dispersion of 0.4mL / h, and a receiving distance of 18cm for electrospinning. After 12 hours, the collected fibers were electrospun with a diameter of 400nm to 1μm. The composite film has a thickness of 10 μm to 50 μm, and the chitosan gel content is 0.2% to 0.3%. Taking HRP as a drug model, the encapsulation rate is above 70% (mass), and the burst release rate is below 20%, achieving the purpose...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com