Substitutive (S)-benaldehyde sulfonyl pyrrolidine-3-amino derivative, and preparation method and application thereof
A methyl and double-substituted technology, applied in the field of substituted (S)-phenylmethylsulfonylpyrrolidine-3-amino derivatives and their preparation and application, can solve problems such as the difficulty of finding dual inhibitors, and achieve structural The effect of correctness, simple reaction, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
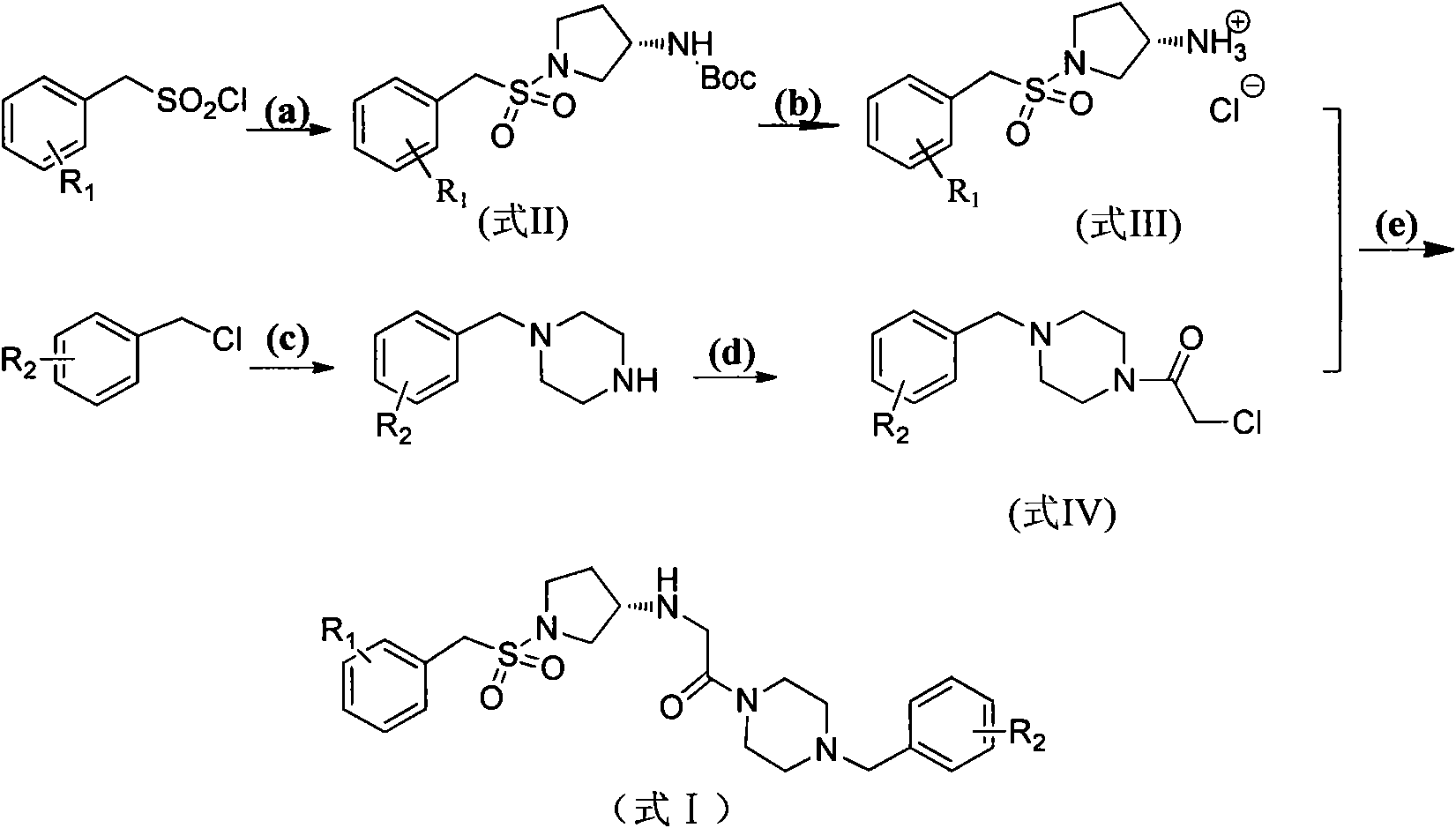
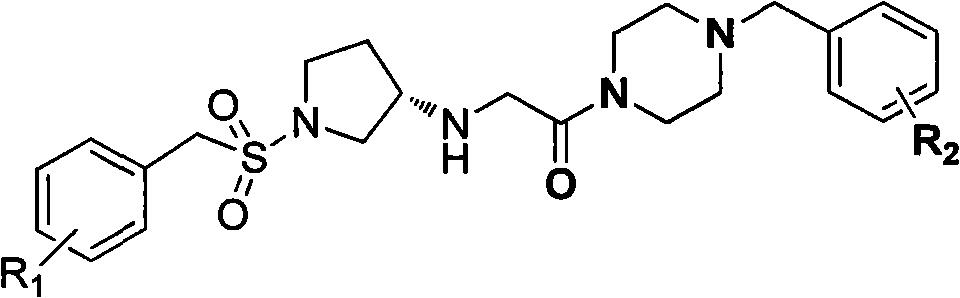
[0042] Example 1, (S)-2-[(1-benzylsulfonyl)pyrrolidine-3-amino]-1-(4-benzylpiperazine)ethanone (in formula I, R1 is H, R2 is Compounds of H) Synthesis
[0043] Step (1): Dissolve 60 mmol potassium carbonate in 20 mL water and stir until completely dissolved, add 30.6 mmol (S)-Boc-3-aminopyrrolidine in ethyl acetate (70 mL), and stir at room temperature for 25 minutes. 33mmol of benzylsulfonyl chloride was dissolved in 60mL of ethyl acetate and tetrahydrofuran (1:1, v / v), added dropwise under an ice-water bath, and reacted at room temperature for one day after dropping. The reaction solution was concentrated under reduced pressure, then diluted with 30 mL of water and 50 mL of ethyl acetate, and then the organic phase was separated and collected, washed successively with saturated sodium bicarbonate solution, water, and saturated brine, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain Yellow solid (S)-tert-butyl-1-benzylsulfonylpy...
Embodiment 2
[0051] Example 2, Preparation of differently substituted (S)-phenylmethylsulfonylpyrrolidine-3-amino derivatives
[0052] According to the method of Example 1, replace "benzylsulfonyl chloride" in step 1) with "substituted benzylsulfonyl chloride", and replace "benzylpiperazine" in step 3) with "substituted benzylpiperazine" The following corresponding substituted (S)-benzenemethylsulfonylpyrrolidine-3-amino derivatives can be obtained.
[0053] (S)-2-[(1-Benzylsulfonyl)pyrrolidine-3-amino]-1-[4-(2-methylbenzyl)piperazine]ethanone
[0054] The structural confirmation data are as follows: 1 H NMR (400MHz, CDCl 3 ) δ7.41-7.43 (m, 2H), δ7.35-7.39 (m, 3H), δ7.30 (d, J=7.2Hz, 2H), δ7.14-7.21 (m, 3H), δ4. 25(s, 2H), δ3.61(br s, 2H), δ3.48(s, 2H), δ3.34-3.38(m, 1H), δ3.31-3.33(m, 4H), δ3. 28(d, J=6.0Hz, 2H), δ3.27(br s, 1H), δ2.96-3.00(m, 1H), δ2.44(t, J=4.8Hz, 4H), δ2.37 (s, 3H), δ1.96-2.04 (m, 2H), δ1.73 (dt, J=18.0, 6.4Hz, 1H); 13 C NMR (100.6MHz, CDCl 3 )δ168.75,137.60,135...
Embodiment 3
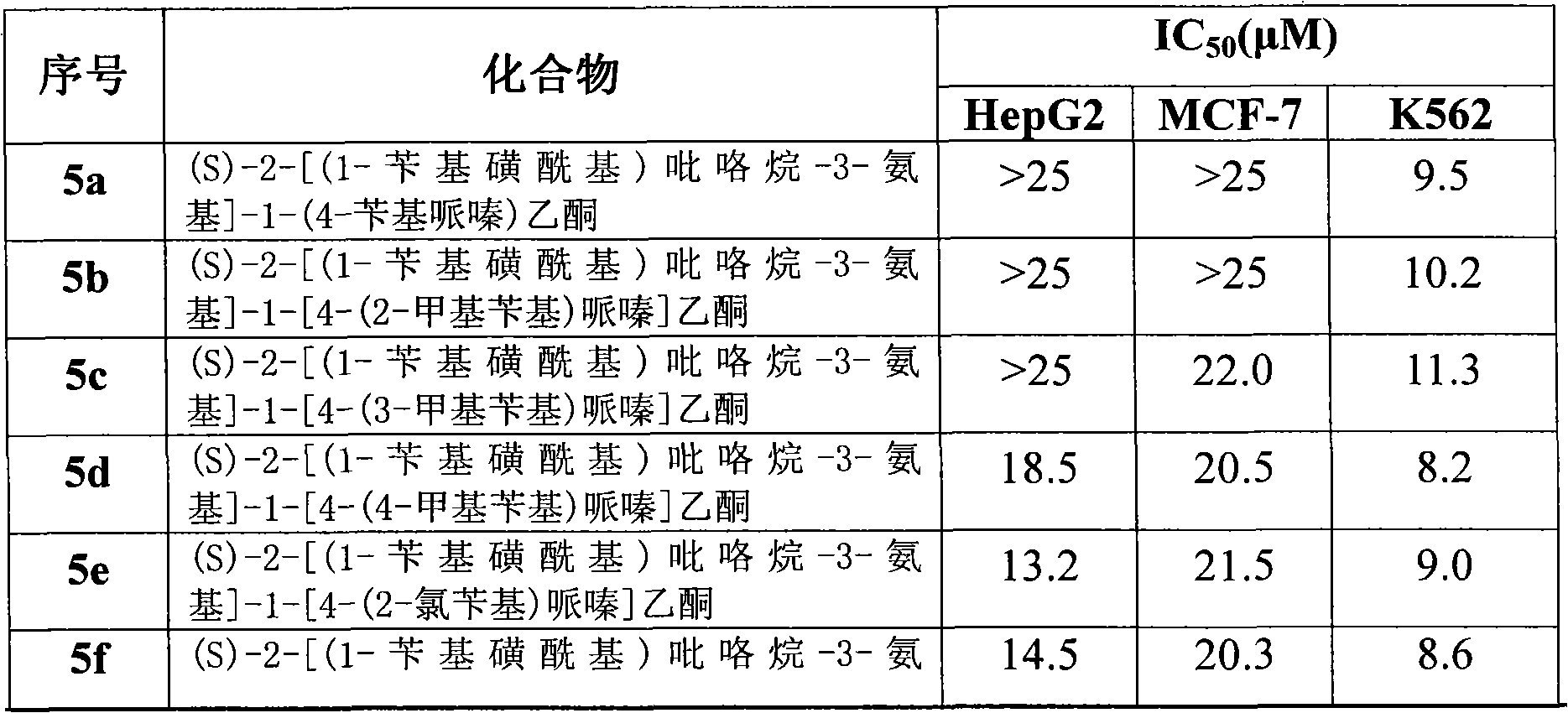
[0103] Example 3, MTT method cell proliferation inhibitory activity screening
[0104] Human liver cancer cell HepG-2 (catalogue number: TChu 72), breast cancer cell MCF-7 (catalogue number: TCHu 74) and leukemia cell K562 (catalogue number: TCHu191) in logarithmic growth phase were taken, (the above cells were purchased from In the Cell Bank of the Typical Culture Collection Committee of the Chinese Academy of Sciences, and the Cell Resource Center of the Shanghai Institutes for Biological Sciences, the Chinese Academy of Sciences) with 2×10 5 Seed / mL density in 96-well plate, 99 μL / well, at 37 ° C, 5% CO 2 After culturing in the incubator for 4 hours, the compounds prepared in the examples of the present invention were added to each well so that the final concentrations were 50 μmol / L, 25 μmol / L, 10 μmol / L, 5 μmol / L, 2.5 μmol / L, 1 μmol / L, 10 concentration gradients of 0.5μmol / L, 0.25μmol / L, 0.1μmol / L, 0.05μmol / L. Three replicate wells were set up for each compound, and bot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com