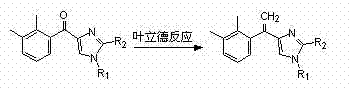
Synthetic method of 4-[1-(2,3-dimethylphenyl)vinyl-1-R1-2-R2 imidazole
A technology of dimethylphenyl and synthetic method, which is applied in the application field of the synthesis of important intermediates of medetomidine, can solve the problems of cumbersome operation, expensive reagents, unfavorable industrial production, etc., and achieve mild reaction conditions and high yield good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] In a 500 mL three-necked flask, add 0.1 mol of n-butyllithium solution and 200 mL of anhydrous tetrahydrofuran, add 35.7 g of triphenylmethylphosphorus bromide under stirring, and then stir at room temperature for 4 hours. Next, 20 g of 2,3-dimethylphenylimidazol-4-one was added with stirring. After the addition was complete, the reaction was stirred overnight. After the reaction was completed, it was filtered, and the filtrate was concentrated to dryness under reduced pressure. The concentrate was purified by silica gel column (eluent: 5% to 15% by weight methanol dichloromethane) to obtain 17.6g of 4-[1-(2,3-dimethylphenyl)vinyl]imidazole .
Embodiment 2
[0016] In a 500 mL three-necked flask, add 0.1 mol of n-butyl lithium solution and 300 mL of anhydrous tetrahydrofuran, add 35.7 g of triphenylmethylphosphonium bromide under stirring, and then stir at room temperature for 4 hours. Then 44.2 g of 2,3-dimethyl-1-(trityl)imidazol-4-one were added with stirring. After the addition was complete, the reaction was stirred overnight. After the reaction was completed, it was filtered, and the filtrate was concentrated to dryness under reduced pressure. Purify the concentrate through a silica gel column (eluent is petroleum ether ethyl acetate with a concentration of 10% to 20% by weight) to obtain 41 g of 4-[1-(2,3-dimethylphenyl)vinyl]-1 -(trityl)imidazole.
Embodiment 3
[0018] In a 500 mL three-necked flask, add 0.1 mol of n-butyllithium solution and 300 mL of anhydrous tetrahydrofuran, add 35.7 g of triphenylmethylphosphonium bromide under stirring, and then stir at room temperature for 4 hours. Then add 42.1 g of 2,3-dimethylphenyl-1-dimethylsulfonamido-2-tert-butyldimethylsilyl imidazol-4-one under stirring, and the reaction is stirred overnight . After the reaction was completed, it was filtered, and the filtrate was concentrated to dryness under reduced pressure. Purify the concentrate through a silica gel column (eluent is petroleum ether ethyl acetate with a concentration of 5% to 10% by weight) to obtain 35.2g of 4-[1-(2,3-dimethylphenyl)vinyl] -1-Dimethylsulfonamido-2-tert-butyldimethylsilylimidazole.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com