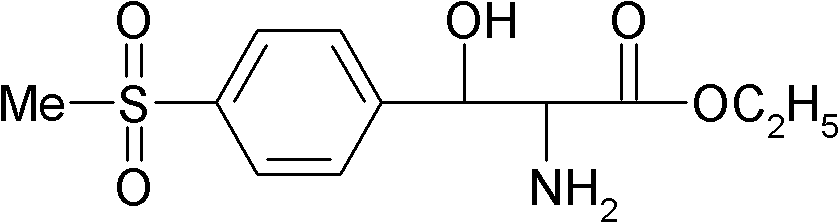
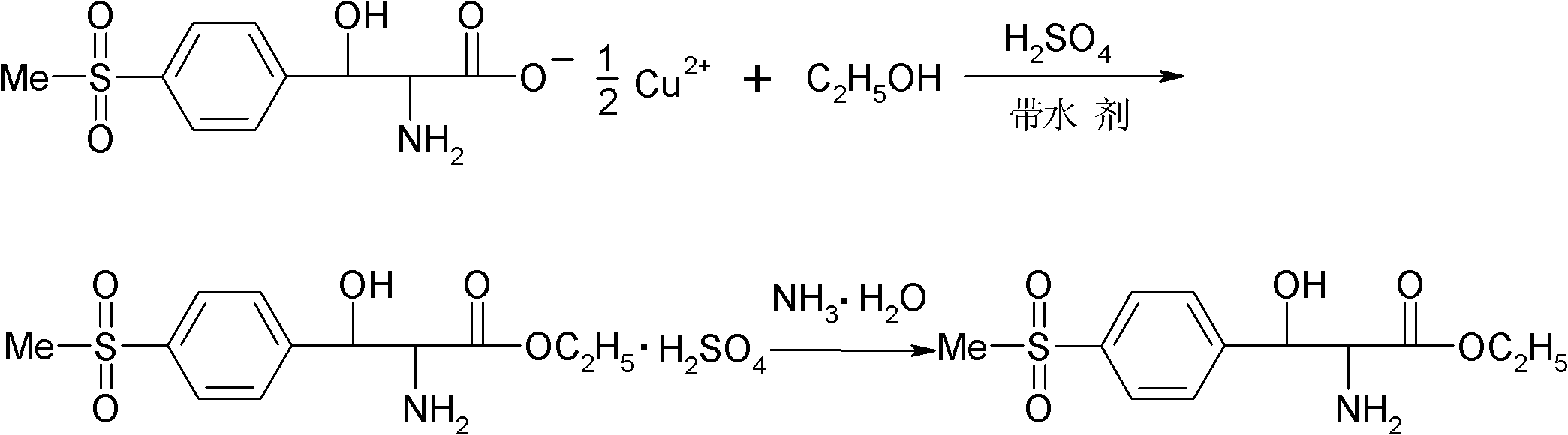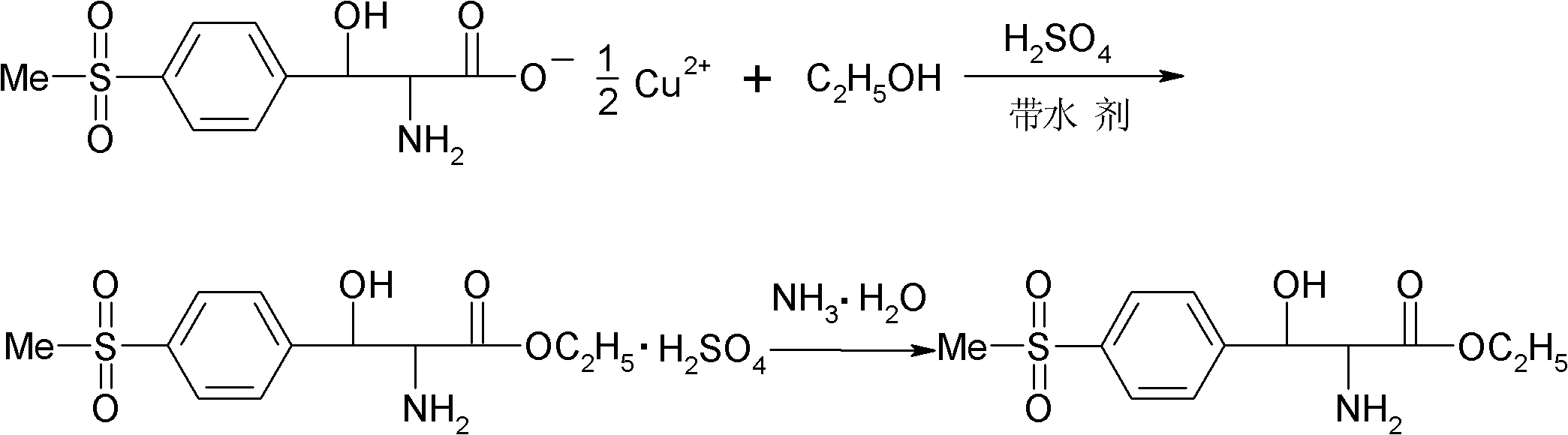Preparation method of DL-p-methylsulfonylphenyl serine ethyl ester
The technology of methylsulfonyl phenylserine ethyl ester and methylsulfonyl phenylserine copper is applied in the field of preparation of DL-p-methylsulfonyl phenylserine ethyl ester, and can solve the problems of strong corrosiveness, high cost, low product yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Esterification: Add 120 g of absolute ethanol and 35 g of benzene in a four-necked flask equipped with mechanical stirring, reflux condenser and thermometer, slowly add 60 g of concentrated sulfuric acid dropwise at room temperature, and add 58 g of p-thiamphenicol after the addition is completed. Phenylserine copper salt, warm to reflux. During the reflux reaction process, the moisture generated by the reaction was continuously removed through the water separator, and the reflux reaction was performed for 30 hours. After the reaction was completed, the temperature was slightly lowered while hot and the filter cake was copper sulfate. The filtrate was placed at 0°C overnight for crystallization, and finally filtered by suction to obtain DL-p-thymphenylphenylserine ethyl sulfate.
[0026] (2) Dissociation and neutralization: Add 75g of deionized water into a four-neck flask equipped with stirring, reflux condenser and thermometer, and add the above-mentioned DL-p-thy...
Embodiment 2
[0028] (1) Esterification: Add 120 g of absolute ethanol and 70 g of toluene to a four-necked flask equipped with mechanical stirring, reflux condenser and thermometer, slowly add 60 g of concentrated sulfuric acid dropwise at room temperature, and add 58 g of p-thiamphenicol after the addition is completed. Phenylserine copper salt, warm to reflux. During the reflux reaction process, the moisture generated by the reaction was continuously removed through the water separator, and the reflux reaction was carried out for 18 hours. After the reaction was completed, the temperature was slightly lowered while hot and the filter cake was copper sulfate. The filtrate was placed at 0°C overnight for crystallization, and finally filtered by suction to obtain DL-p-thymphenylphenylserine ethyl sulfate.
[0029] (2) Dissociation and neutralization: Add 75g of deionized water into a four-neck flask equipped with stirring, reflux condenser and thermometer, and add the above-mentioned DL-p-t...
Embodiment 3
[0031] (1) Esterification: Add 120 g of absolute ethanol and 46.4 g of xylene in a four-necked flask equipped with mechanical stirring, reflux condenser and thermometer, slowly add 60 g of concentrated sulfuric acid dropwise at room temperature, and add 58 g of p-toluene after the dropwise addition Sulfonylphenylserine copper salt, heated to reflux. During the reflux reaction process, the moisture generated by the reaction was continuously removed through the water separator, and the reflux reaction was performed for 20 hours. After the reaction was completed, the temperature was slightly lowered while hot and the filter cake was copper sulfate. The filtrate was placed at 0°C overnight for crystallization, and finally filtered by suction to obtain DL-p-thymphenylphenylserine ethyl sulfate.
[0032] (2) Dissociation and neutralization: Add 75g of deionized water into a four-neck flask equipped with stirring, reflux condenser and thermometer, and add the above-mentioned DL-p-thy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com