Preparation method of recombinant human corticotropin releasing factor
An adrenal cortex and factor releasing technology, applied in the field of genetic engineering, can solve the problems of steric hindrance, incision, low endoproteinase cleavage efficiency, etc., and achieve the effects of low production cost, improved recovery rate, and improved druggability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
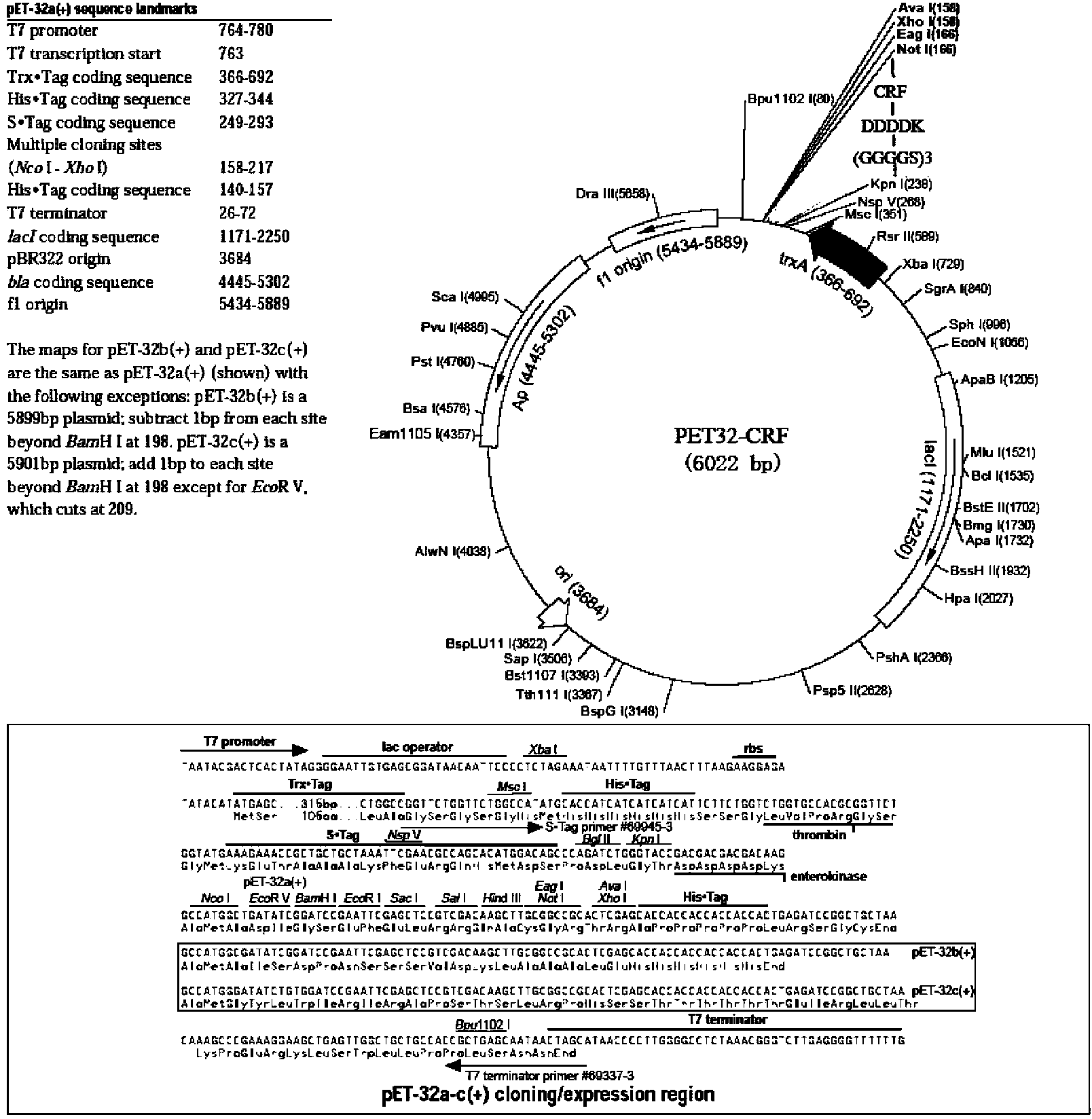
[0041] Example 1 Construction of engineering bacteria expressing rhCRF
[0042] 1. CRF gene design and synthesis
[0043] According to the human CRF amino acid sequence registered in GenBnak (accession number: CAA23834.1), the 41 amino acid gene coding sequence of the mature peptide was selected, and synonymous mutations were carried out taking into account codon bias and gene sequence optimization in E. coli. And at the 5' end of the gene, the nucleic acid coding sequence corresponding to the enterokinase recognition sequence (DDDDK) and the linking peptide (GGGGSGGGGSGGGGS) were sequentially connected, and at the same time, the KpnI recognition sequence GATTCC was introduced at the 5' end of the entire sequence, and the NotI recognition sequence GCGGCCGC was introduced at the 3' end. The designed gene sequence was subjected to whole gene synthesis (completed by Shanghai Sangong).
[0044] 2. Construction of pET32-CRF / BL21(DE3) engineering bacteria
[0045] 2.1 Construction...
Embodiment 2
[0056] Example 2 Fermentation of engineering bacteria pET32-CRF / BL21 (DE3)
[0057] Inoculate the seed solution cultivated overnight into a suitable medium (such as LB, TB or other suitable medium) in the fermenter for cultivation, and culture the bacteria to OD 600 For about 25, add such as IPTG induction 3-4 hours. Collect the bacteria by centrifugation, suspend the bacteria in a suitable buffer solution (25mm Tris-HCl, 150 mmNaCl, pH8.0), homogenize under high pressure to break the bacteria, centrifuge the broken solution, collect the supernatant, and discard the precipitate.
Embodiment 3
[0058] Example 3 rhCRF purification
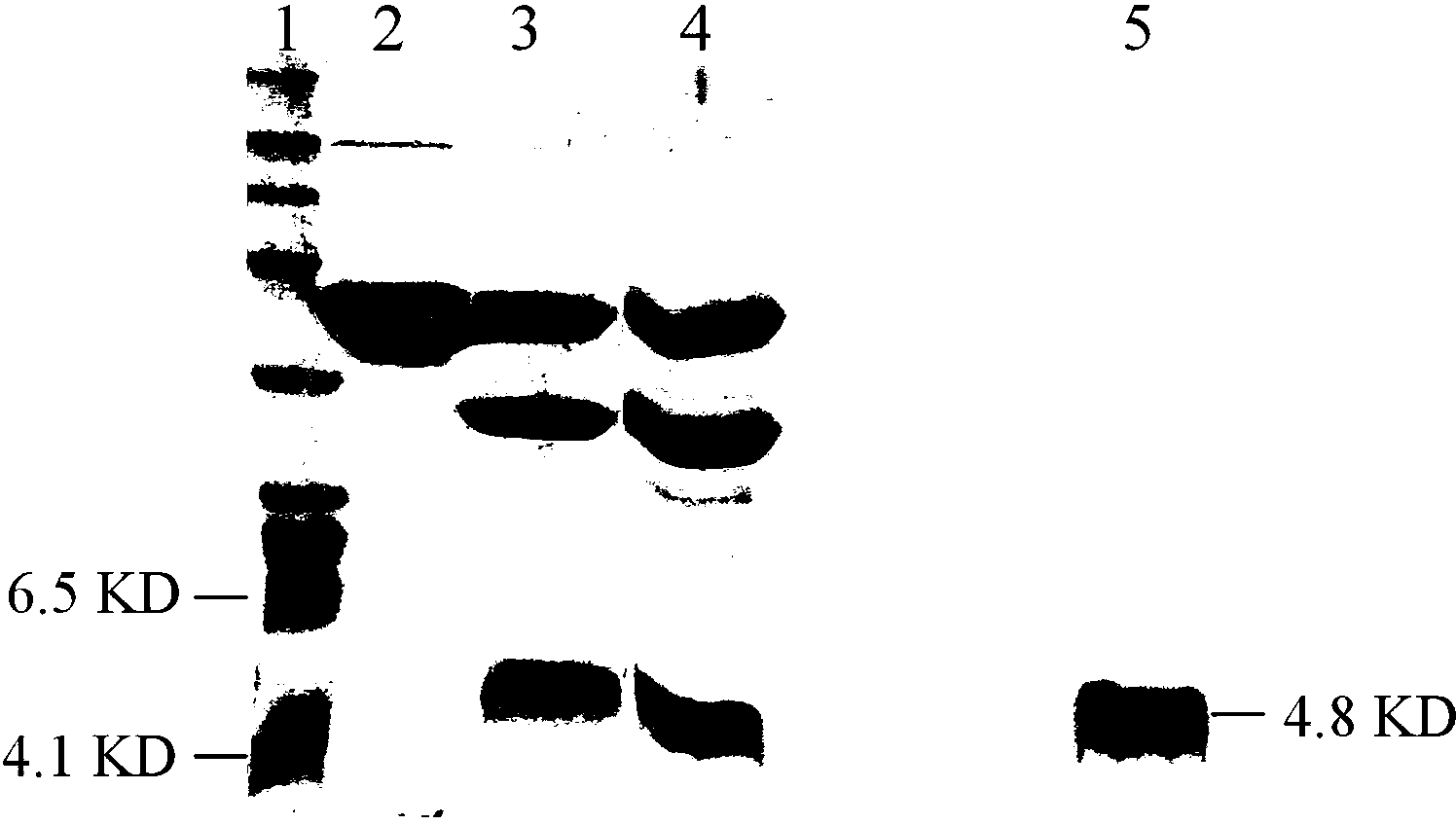
[0059] The bacteriostasis supernatant was loaded on the Ni2+-Chelating Sepharose Fast Flow (GE Healthcare) chelation chromatography medium treated with 0.2M NiSO4 and equilibrated with 20mM Tris-HCl (pH8.0), and 100mM imidazole (containing 20mM Tris-HCl, pH8.0) was eluted; the collected target fusion protein was desalted, and 0.5U enterokinase (50mM Tris-HCl, 2mM CaCl2, 0.1% Tween-20, pH8.0) was added to each 1mg fusion protein in Digest the fusion protein for about 16 hours at 4°C.
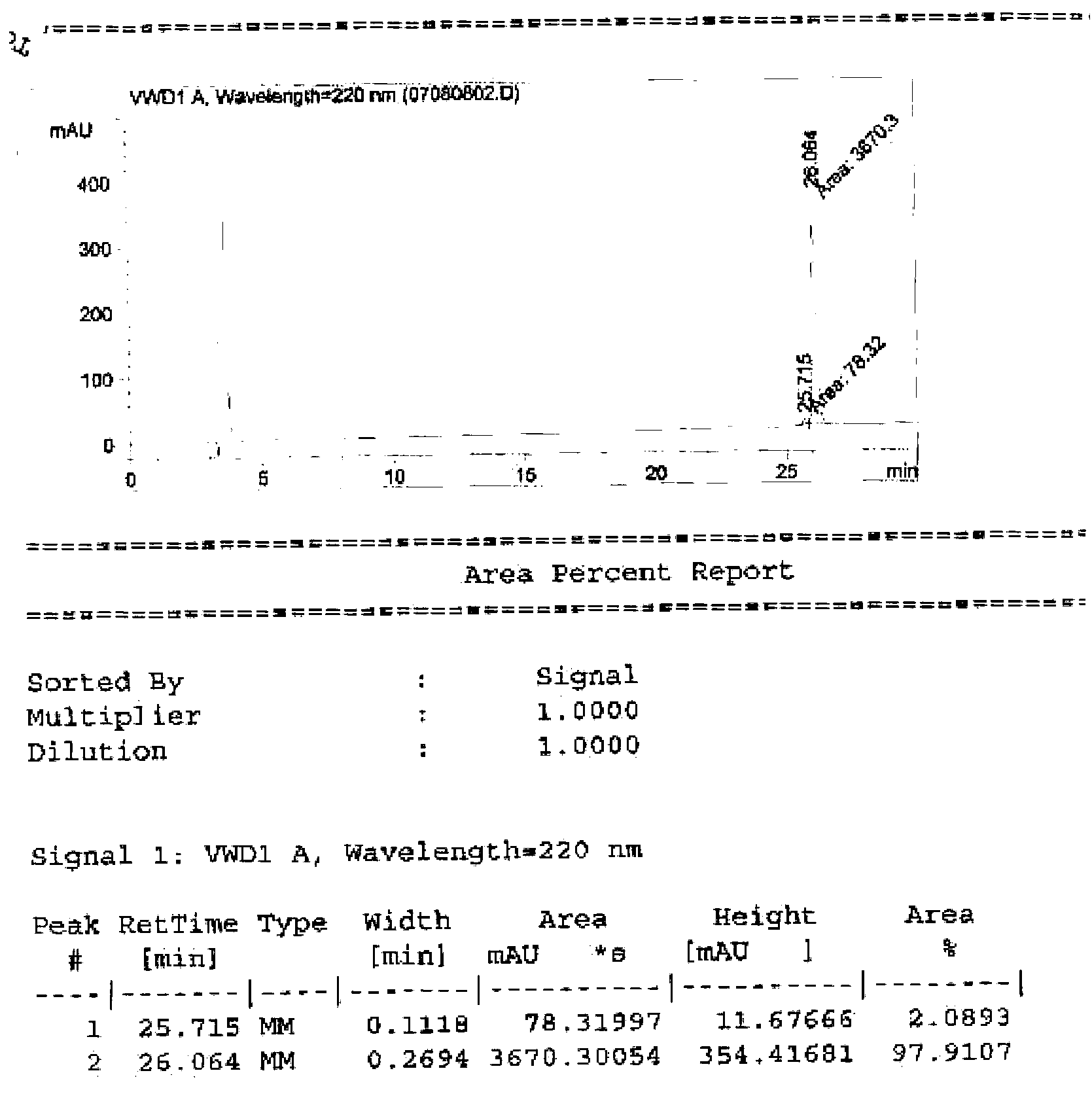
[0060] The digested target protein is loaded on Ni2+-Chelating Sepharose Fast Flow, and the target protein and fusion tag are simultaneously hung on the column. However, the binding capacity of the target protein on the column is low, and the target protein is eluted with a low concentration of imidazole (50mM), and the peak of the eluted protein is collected. The SDS-PAGE detection molecular weight is about 4700Da, the purity is greater than 95%, and the H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com