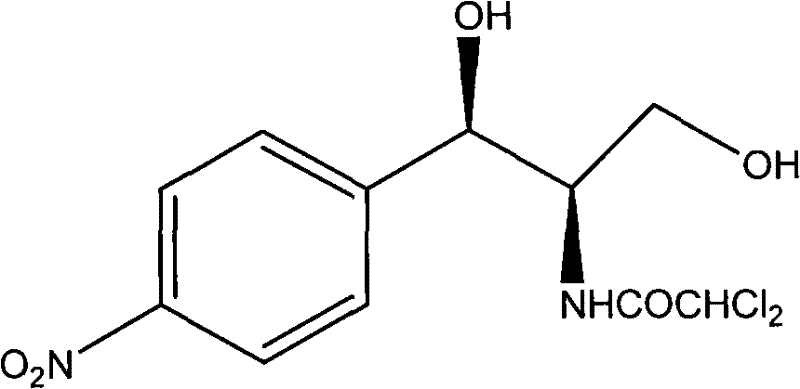
Method for preparing broad-spectrum antibiotic chloramphenicol
A technology of chloramphenicol and propylene glycol, which is applied in the field of compound preparation, can solve the problems of long synthesis route of chloramphenicol, production cost and increase of three wastes, and achieve the effect of solving the three wastes and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
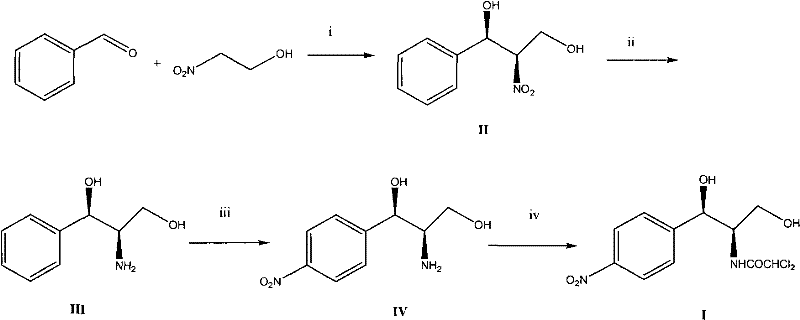
[0028] Embodiment 1 (1R, 2R)-2-nitro-1-phenyl-1, the preparation of 3-propanediol
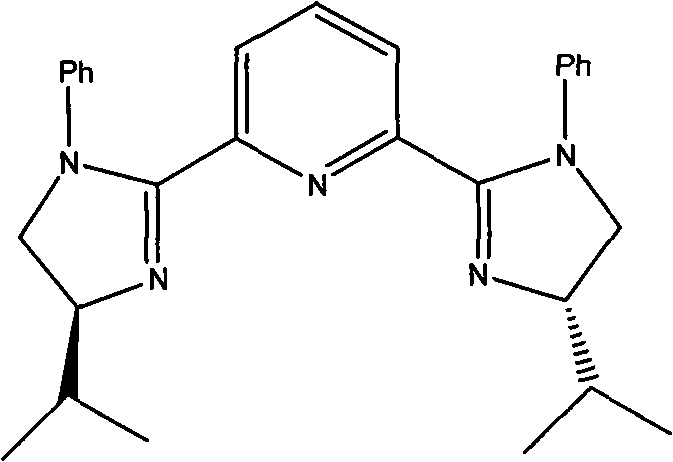
[0029] 0.9 g of Cu(OTf) 2 (0.25mmol), 1.2 grams of 2,6-bis[(S)-4-isopropyl-1-phenyl-4,5-dihydro-1H-2-imidazolyl]pyridine (2.6mmol) and 20 ml Add 1,4-dioxane into a 100 ml single-necked flask, replace the air inside with nitrogen to keep the nitrogen flow constant, stir with magnetic force for 2 hours, then cool with an ice bath, add 2.7 g of benzaldehyde (25 mmol), 22.8 g of 2 -Nitroethanol (250mmol) and N-methylmorpholine (0.27 milliliters, 2.5mmol), the reaction solution was stirred in an ice-bath cooling for 24 hours, after the thin-plate chromatography detected no raw material benzaldehyde spots, then evaporated under reduced pressure to remove volatile non-toxic solvent, the catalyst was removed by silica gel filtration, and the filtrate was concentrated to obtain 4.4 grams of product, the yield was 90%, and the e.e value was 93% as determined by HPLC. 1 H NMR (acetone-d 6 )δ: 3.46(ddd,...
Embodiment 2
[0030] The preparation of embodiment 2 (1R, 2R)-2-amino-1-phenyl-1,3-propanediol
[0031] Dissolve 5 g of (1R, 2R)-2-nitro-1-phenyl-1,3-propanediol (25 mmol) in 100 ml of methanol, add 0.1 g of 10% palladium-on-carbon catalyst, under hydrogen pressure 50Psi Hydrogenation reduction, no raw material spots detected by thin-plate chromatography, and the catalyst was removed by filtration. After the filtrate was concentrated, it was recrystallized with a 1:1 ethanol-ether mixed solvent to obtain 4.0 g of the product, with a yield of 94%, m.p.111-113 ° C, other data and literature to , Y.; Masaya, S.; Hayashi, T. J. Am. Chem. Soc. 1986, 108, 6405. Concordance.
Embodiment 3
[0032] Embodiment 3 (1R, 2R)-2-amino-1-p-nitrophenyl-1, the preparation of 3-propanediol
[0033] Slowly add 4 grams of (1R,2R)-2-amino-1-phenyl-1,3-propanediol (24mmol) into 15ml of concentrated sulfuric acid in batches, cool to -2~0°C, and then add 15ml Nitric acid, stirred at the above temperature for 5 hours, no raw material spots were detected by thin-plate chromatography, and the reaction solution was poured into 500 grams of ice, then carefully neutralized with 30% aqueous sodium hydroxide solution, extracted three times with dichloromethane, and combined to extract liquid, concentrated after drying, and recrystallized with a small amount of water to obtain 2.8 g of the product, with a yield of 55%, m.p.141-143°C. The NMR spectrum is consistent with the literature Hazra, B.G., Pore, V.S., Maybhate, S.P., Natekar, M.V. and Rao, A.S., Synfh.Commiin., 1989, 19, 1763.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com