Inactivated vaccine for infectious coryza of chickens
A technology for chicken infectious rhinitis and inactivated vaccine, which is applied in the directions of antibacterial drugs, antibody medical ingredients, and medical preparations containing active ingredients, etc., and can solve the problems of short duration, side reactions, and low antibody titers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] (3) Preparation of seedling liquid
[0017] 1) Bacterial liquid culture Add 100ml of seed liquid into 4000ml of semi-synthetic medium, cultivate at 37°C for 18-20 hours, vibrate the culture bottle twice, and confirm that there is no bacterial contamination by pure picking, add 0.05% formaldehyde solution, place Store at 2-8°C.
[0018] 2) Concentration and inactivation of the bacterium liquid for making vaccines Use a hollow fiber ultrafilter to concentrate the purely qualified bacterium liquid, and then make a suspension with PBS of pH 7.2, so that every 1ml contains at least 5 billion bacterium (using biological The product national standard product "China Bacterial Turbidity Standard" is compared, set standard tube containing Haemophilus paragallinarum to be 2,800,000,000 / ml), then add 0.15% formaldehyde solution and 0.01% thimerosal by volume ratio, at 2~8 ℃ Sterilized for 7 days, the aseptic growth was tested as the vaccine antigen.
[0019] (4) Preparation of ad...
Embodiment 1
[0038] 1 strain
[0039] The strain used for manufacturing and inspection is Haemophilus paragallinarum type A C-Hpg-8 strain, which is identified, kept and supplied by China Veterinary Drug Control Institute.
[0040] 2 Vaccine manufacturing and inspection of semi-finished products
[0041] (1) Preparation of seeds for production
[0042] 1) Propagation and identification of primary seeds Take the strains and inoculate them on the chicken broth agar 2 After culturing at 37°C for 16-18 hours in the environment, select several typical bacterial lawns (colonies) with strong fluorescence and inoculate them in the yolk sac of 5-day-old chicken embryos, continue to incubate at 37°C, and collect the yolks of chicken embryos that died within 30 hours The liquid, after passing the pure inspection, is regarded as a first-grade seed. Storage below -20°C should not exceed 1 month, otherwise it will be rejuvenated through chicken embryos, but it should not exceed 6 generations.
[004...
Embodiment 2
[0058] According to " Chicken Infectious Rhinitis Inactivated Vaccine Manufacture and Inspection Regulations " (Ministry of Agriculture of the People's Republic of China. The People's Republic of China Veterinary Biological Products Regulations 2000 Edition. Chemical Industry Press, 2000, the present invention is hereinafter referred to as " Regulations ") Test the vaccine according to the test method above.
[0059] 1. Sterility test According to the "Procedures", aseptic growth.
[0060] 2. Safety inspection Use 8 healthy susceptible chickens aged 2-3 months, inject 1ml of vaccine subcutaneously into each chicken, observe for 14 days, there should be no abnormal reaction.
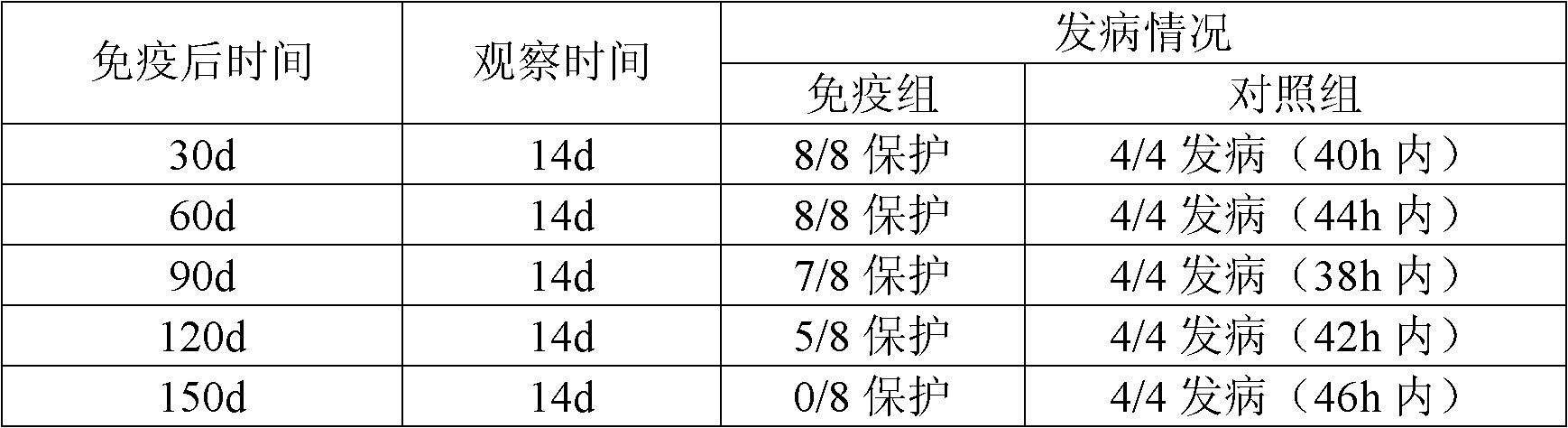
[0061] 3. Efficacy test Eight healthy susceptible chickens aged 2 to 3 months were used, and each chicken was subcutaneously injected with 0.5ml of the vaccine. One month later, together with 4 control chickens with the same conditions, each infraorbital sinus was injected with 0.2ml of a 16-hour culture...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com