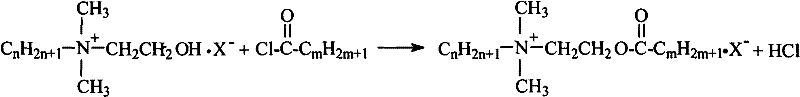Double-long chain ester-based quaternary ammonium salt and synthesis technology thereof
A technology of ester quaternary ammonium salt and synthesis process, which is applied in the preparation of amino hydroxy compounds, the preparation of organic compounds, organic chemistry, etc. Gentle, no three wastes, easy to separate and purify
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Weigh 8.91g (0.1mol) of N, N-dimethylethanolamine and 29.91g (0.12mol) of 1-bromododecane, add them to a 250mL three-necked flask, add 45mL of ethanol as a solvent, and put them in a water bath Heat to 85°C for 5 hours to stop the reaction at a constant temperature, remove ethanol by rotary evaporation, and recrystallize with acetone-methanol mixed solvent to obtain dodecyl dimethyl hydroxyethyl ammonium bromide as a white crystal substance; weigh dodecyl dimethyl hydroxyethyl ammonium bromide; Add 16.92g (0.05mol) of ethylammonium bromide into a 250mL three-necked flask, add an appropriate amount of solvent chloroform and acid agent triethylamine, slowly add 15.15g (0.05mol) of stearyl chloride in an ice-water bath, drop After the addition, the temperature was naturally raised to room temperature for 8 hours, and the reaction was stopped. The solvent was removed by rotary evaporation, and the product was recrystallized three times from absolute ethanol to ob...
Embodiment 2
[0022] Embodiment 2: Weigh 8.91g (0.1mol) N, N-dimethylethanolamine and 29.91g (0.12mol) 1-bromododecane, join in 250mL three-necked flask, add 50mL acetone as solvent, water bath Heating to 60°C for 5 hours to stop the reaction at a constant temperature, remove acetone by rotary evaporation, and recrystallize with acetone-methanol mixed solvent to obtain dodecyl dimethyl hydroxyethyl ammonium bromide as a white crystal substance; weigh dodecyl dimethyl hydroxyethyl ammonium bromide; Add 16.92g (0.05mol) of ethylammonium bromide into a 250mL three-necked flask, add an appropriate amount of solvent chloroform and acid agent triethylamine, slowly add 15.15g (0.05mol) of stearyl chloride in an ice-water bath, drop After the addition, the temperature was naturally raised to room temperature for 8 hours, and the reaction was stopped. The solvent was removed by rotary evaporation, and the product was recrystallized three times from absolute ethanol to obtain a white solid product, e...
Embodiment 3
[0023] Example 3: Weigh 8.91g (0.1mol) of N, N-dimethylethanolamine and 33.27g (0.12mol) of 1-tetradecane bromide, add them to a 250mL three-necked flask, add 45mL of ethanol as a solvent, and put them in a water bath Heat to 85°C for 5 hours to stop the reaction at a constant temperature, remove ethanol by rotary evaporation, and use acetone-methanol mixed solvent to recrystallize to obtain tetradecyl dimethyl hydroxyethyl ammonium bromide as a white crystal substance; weigh tetradecyl dimethyl hydroxyethyl ammonium bromide; Add 18.32g (0.05mol) of ethylammonium bromide into a 250mL three-necked flask, add an appropriate amount of solvent chloroform and acid agent triethylamine, slowly add 15.15g (0.05mol) of stearyl chloride in an ice-water bath, drop After the addition, the temperature was naturally raised to room temperature for 8 hours, and the reaction was stopped. The solvent was removed by rotary evaporation, and the product was recrystallized three times with absolute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com