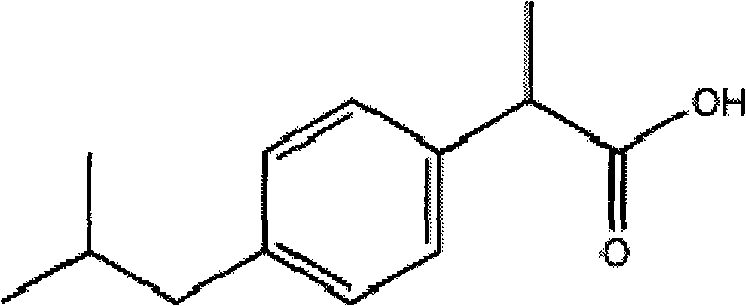
Stable ibuprofen arginine injection and preparation method thereof
An injection, arginine technology, applied in the field of pharmaceutical preparations, can solve the problems of white opalescence or turbidity, side effects, unfavorable intravenous injection or intramuscular injection, etc., to reduce irritation, reduce production costs, and solve the problems of chemical stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
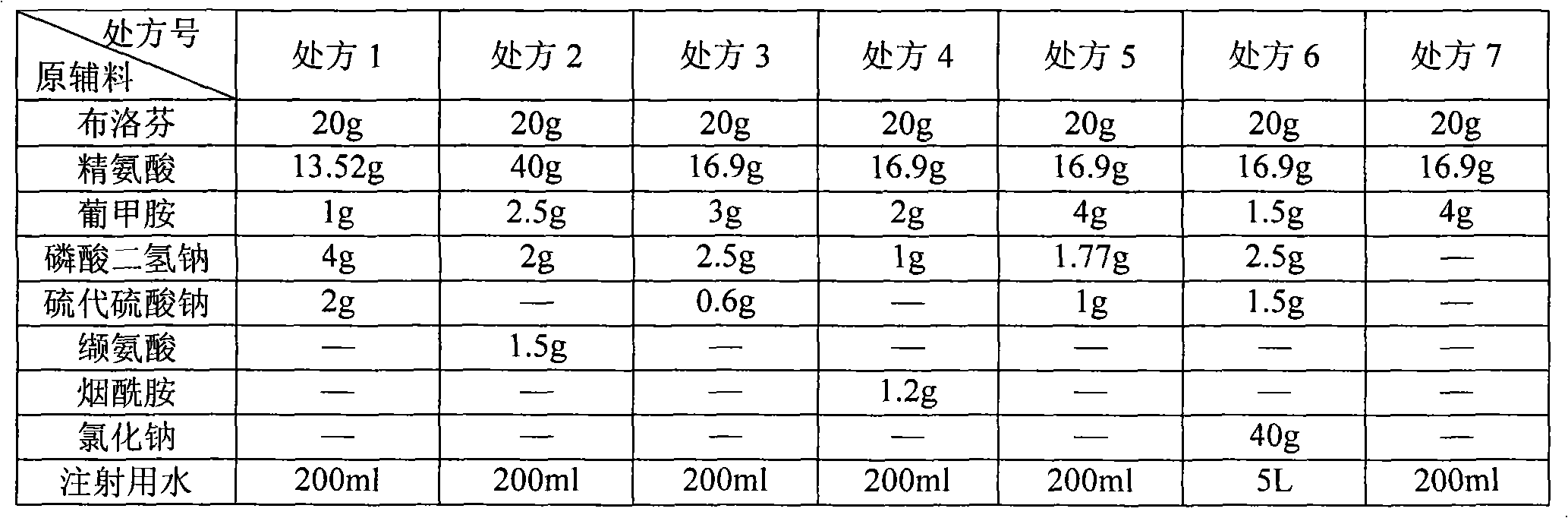
[0045] See Table 1 for the formula (prescription) of ibuprofen arginine injection.
[0046] Table 1 Prescription List of Ibuprofen Amino Acid Injection
[0047]
[0048] Preparation Process:
[0049] 1. Weigh the prescribed amount of arginine in a beaker, add 80% water for injection (wherein, prescription 6 adds 1% water for injection), stir to make it dissolve, and form a solution;
[0050] 2. Add ibuprofen into the solution while stirring until completely dissolved;
[0051] 3. Add antioxidant, meglumine, sodium dihydrogen phosphate, and sodium chloride (prescription 6) into the solution, stir to dissolve completely;
[0052] 4. Measure the pH value of the solution, adjust the pH to 7.0-8.0 with hydrochloric acid or sodium hydroxide if necessary, and add water for injection to the configured volume;
[0053] 5. Add 0.5% activated carbon and stir for 30 minutes;
[0054] 6. First use 0.45μm filter to decarbonize, and then use 0.22μm fine filter to obtain the filtrate; ...
Embodiment 2
[0059] Stability test:
[0060] The sample is subjected to the influence factor test, and the changes of related substances and colors of the sample before and after the influence factor are measured.
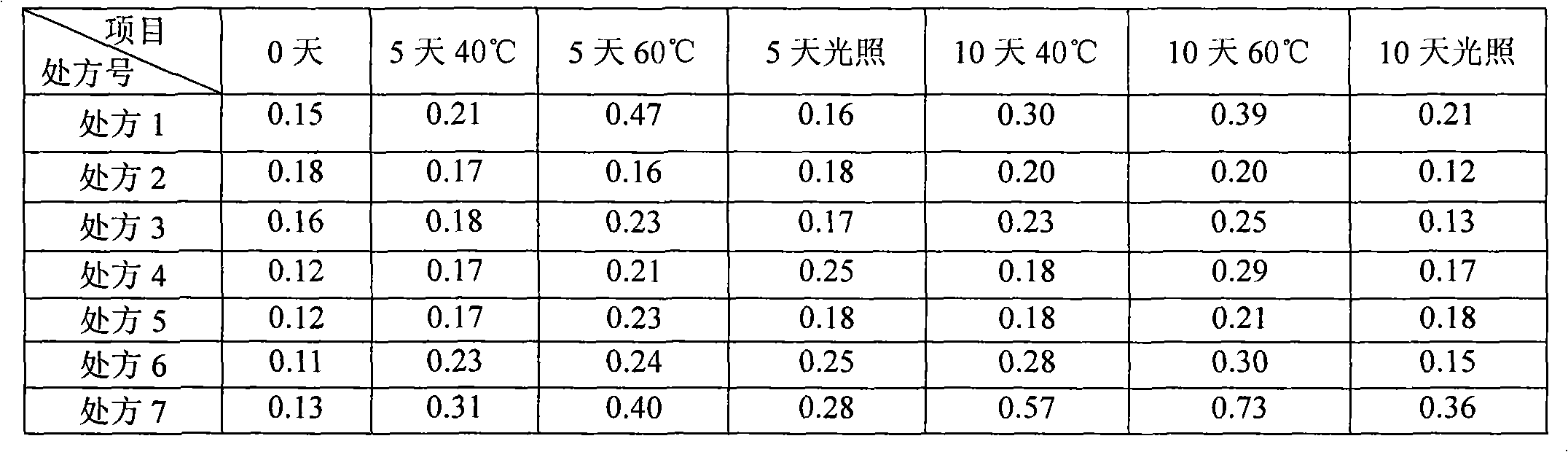
[0061] The samples of the ibuprofen arginine injection prepared by the above 6 prescriptions were placed in the light, 40°C and 60°C influencing factor test chambers for investigation, and the related substances and color changes were measured on the 5th and 10th days respectively. And compared with 0 days, the specific data are shown in Table 2 and Table 3.
[0062] Table 2 Influencing factors Experimental related substances (%)
[0063]
[0064] Table 3 Influencing factors Experimental color
[0065]
[0066] Conclusion: The color of the prescription without antioxidant (prescription 7) turns yellow obviously at 40°C and 60°C, and the related substances also increase significantly. The samples prepared by other prescriptions and processes of the present invention have...
Embodiment 3
[0068] Clarity test after sample dilution
[0069] Take the above-mentioned samples of prescription 1 to prescription 5 and prescription 7 (among them, prescription 5 is the sample reconstituted by adding an equal volume of water for injection before freeze-drying) and take 4ml, dilute with 100ml water for injection and 100ml 5% glucose injection respectively Afterwards, observe the clarity of the solution and check with Caldolor TM For comparison, the specific results are shown in Table 4.
[0070] Table 4 Clarity after sample dilution
[0071]
[0072] Conclusion: after the sample that adopts prescription and technology preparation of the present invention only needs to be diluted with 100ml normal saline and 100ml 5% glucose injection, the solution can be clarified, and Caldolor TM It needs to be diluted with at least 250ml of normal saline to clarify, and 250ml of 5% glucose injection still cannot make the solution clear. Since the commonly used clinical saline and 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com