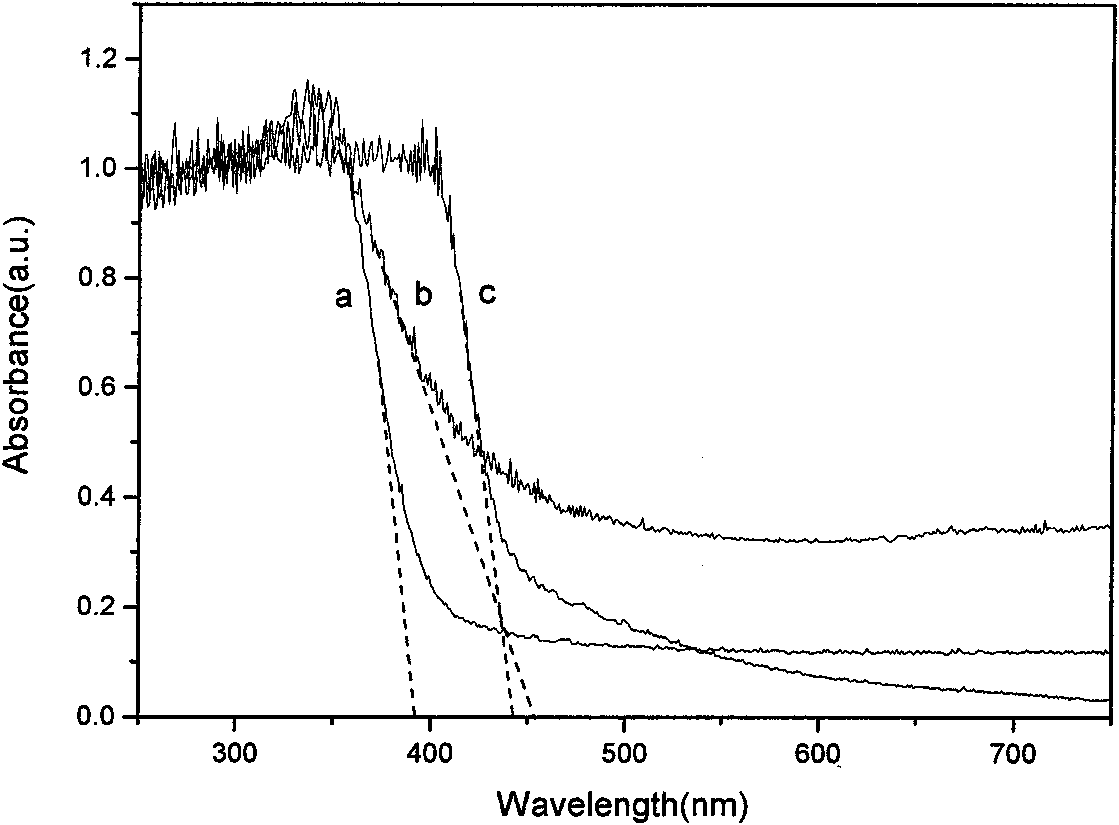
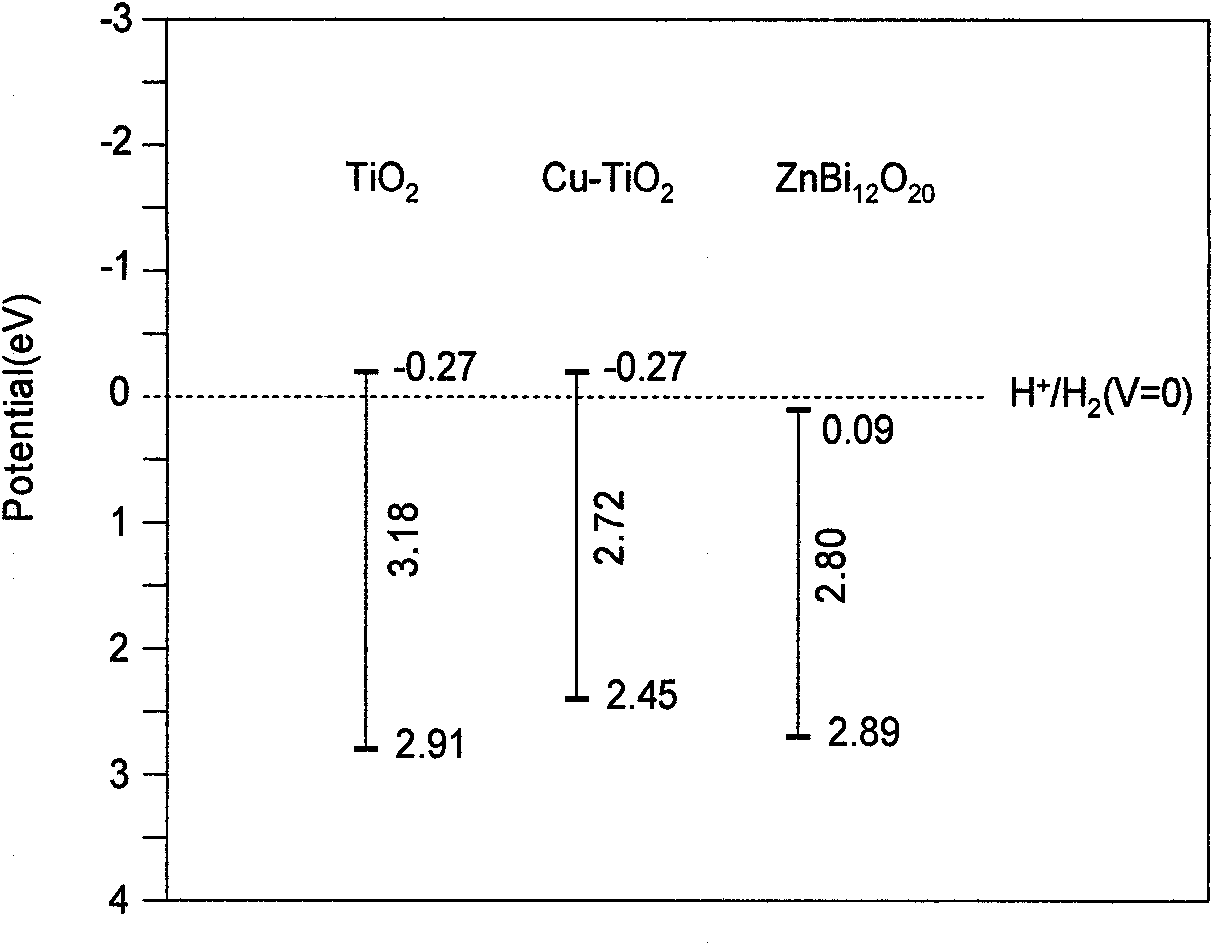
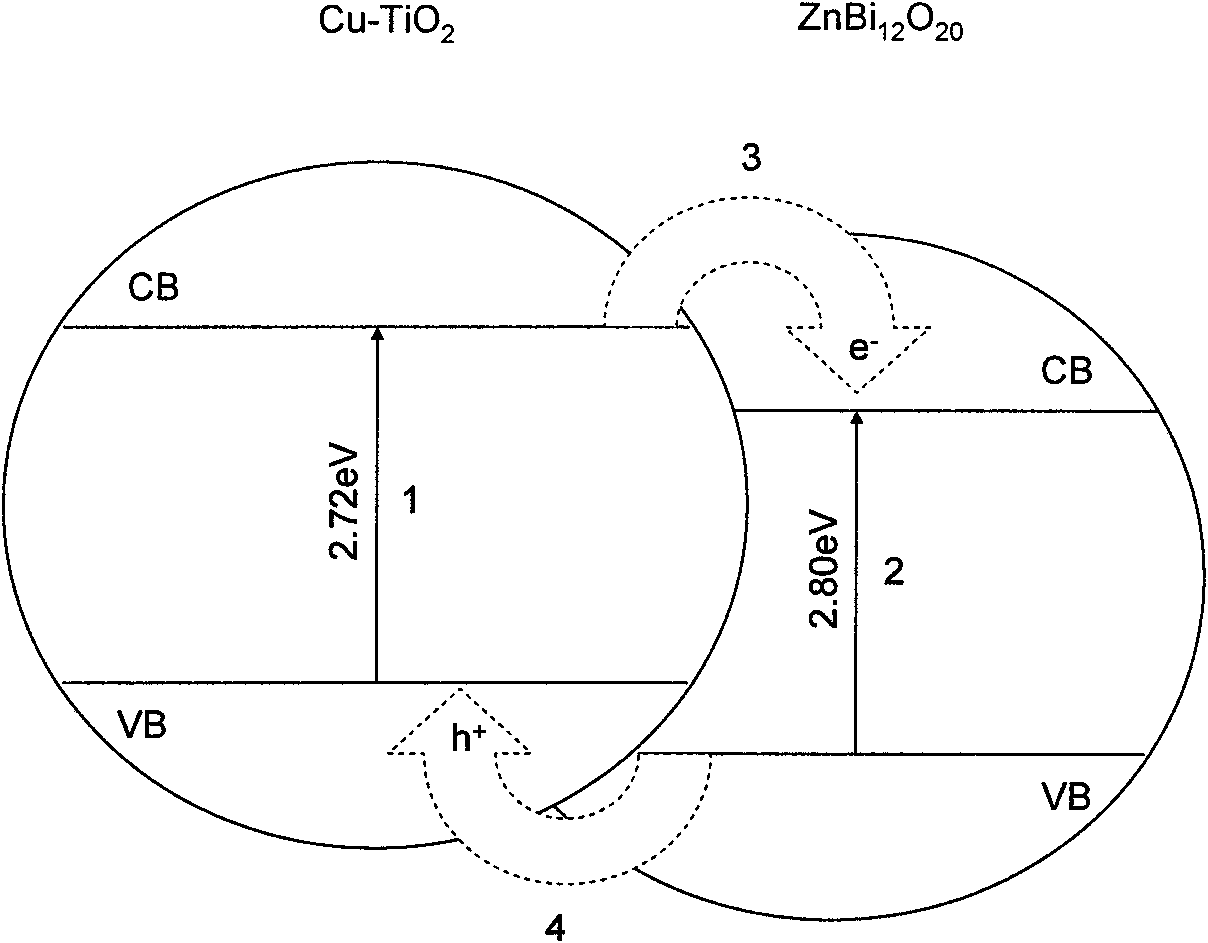
A cu-doped tio2-coupled semiconductor photocatalyst, preparation method and application
A photocatalyst and semiconductor technology, used in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of small number of photocatalysts, narrow band gap, and no visible light activity. and other problems, to achieve the effect of expanding the spectral response range and improving the catalytic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1. Preparation of 20g Cu-TiO 2 Cu doping amount is 0.6wt%, Cu-TiO 2 / ZnBi 12 o 20 Medium Cu-TiO 2 3.0Wt% Cu-TiO 2 / ZnBi 12 o 20 . Its preparation method is: under constant stirring, dissolve 0.0137g Cu(NO 3 ) 2 ·3H 2 The mixed solution of O deionized water, 0.5mL65%~68% nitric acid and 5.0mL absolute ethanol was dropped into the mixed solution of 2.5566g tetrabutyl titanate and 7.8mL absolute ethanol, and 19.4g ZnBi was added 12 o 20 Powder (ZnBi 12 o 20 For the preparation, see 2) in Example 1. After drying in a water bath at 70°C, put it into a muffle furnace and bake at 450°C for 3 hours, and cool to room temperature for storage.
[0019] 2. Prepared ZnBi 12 o 20 . The preparation method refers to the literature (Junwang Tang, Jinhua Ye "Photocatalytic and photophysical properties of visible-Light-driven photocatalyst ZnBi 12 o 20 ""Chemical Physics Letters" 2005, 410: 104~107), that is, to accurately weigh Bi according to the stoichiometric ratio ...
Embodiment 2
[0035] 1. Preparation of 20g Cu-TiO 2 Cu doping amount is 0.6wt%, Cu-TiO 2 / ZnBi 12 o 20 Medium Cu-TiO 2 4.0Wt% Cu-TiO 2 / ZnBi 12 o 20 . Its preparation method is: under continuous stirring, dissolve 0.0182g Cu(NO 3 ) 2 ·3H 2 The mixed solution of O deionized water, 0.68mL65%~68% nitric acid and 6.7mL absolute ethanol was dropped into the mixed solution of 3.4088g tetrabutyl titanate and 10.4mL absolute ethanol, and 19.2g ZnBi was added 12 o 20 Powder (ZnBi 12 o 20 The preparation is the same as 2) in Example 1. After drying in a water bath at 70°C, put it into a muffle furnace and bake at 450°C for 3 hours, and cool to room temperature for storage.
[0036] 2. Preparation of pure TiO 2 . Preparation method is the same as 1 in embodiment two, just do not add Cu(NO 3 ) 2 ·3H 2 O and ZnBi 12 o 20 .
[0037] 3. The activity evaluation of the photocatalyst is the same as 5 in Example 1.
[0038] Under UV light irradiation, 20g4.0Wt%Cu-TiO 2 / ZnBi 12 o 20 T...
Embodiment 3
[0044] 1. Preparation of 20g Cu-TiO 2 Cu doping amount is 0.6wt%, Cu-TiO 2 / ZnBi 12 o 20 Medium Cu-TiO 2 5.0Wt% Cu-TiO 2 / ZnBi 12 o 20 . Its preparation method is: under constant stirring, dissolve 0.0228g Cu(NO 3 ) 2 ·3H 2 The mixed solution of O deionized water, 0.85mL65%~68% nitric acid and 8.3mL absolute ethanol was dropped into the mixed solution of 4.2610g tetrabutyl titanate and 13.0mL absolute ethanol, and 19.0g ZnBi was added 12 o 20 Powder (ZnBi 12 o 20 The preparation is the same as 2) in Example 1. After drying in a water bath at 70°C, put it into a muffle furnace and bake at 450°C for 3 hours, and cool to room temperature for storage.
[0045] 2. Preparation of pure TiO 2 . Preparation method is the same as 1 in embodiment three, just do not add Cu(NO 3 ) 2 ·3H 2 O and ZnBi 12 o 20 .
[0046] 3. The activity evaluation of the photocatalyst is the same as 5 in Example 1.
[0047] Under ultraviolet light irradiation, 20g5.0Wt%Cu-TiO 2 / ZnBi 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| band gap | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com