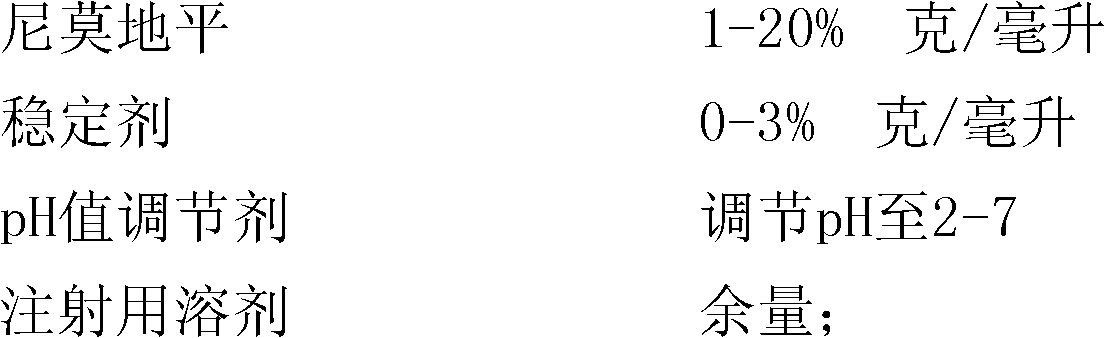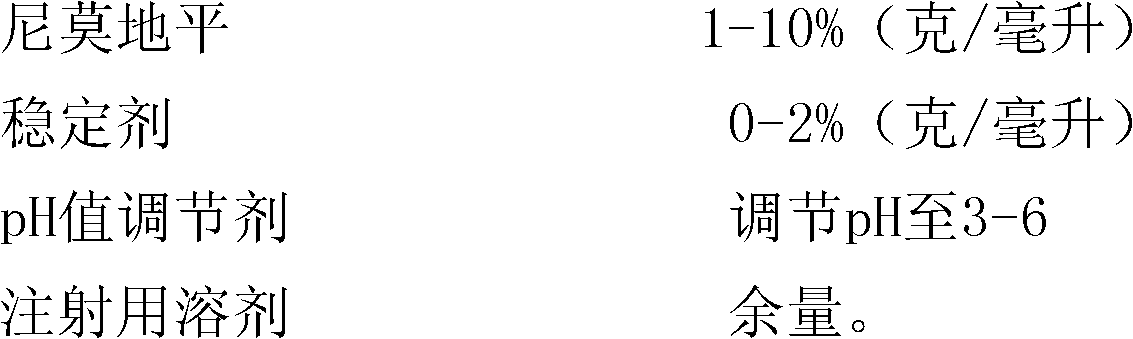Nimodipine injection composition, and preparation method and application thereof
A technology for nimodipine injection and composition, applied in the field of nimodipine injection composition and preparation thereof, can solve the problems of poor cyclodextrin biocompatibility, poor industrialization, long production cycle and the like, and achieve stable Good sex, convenient medication, large drug loading effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
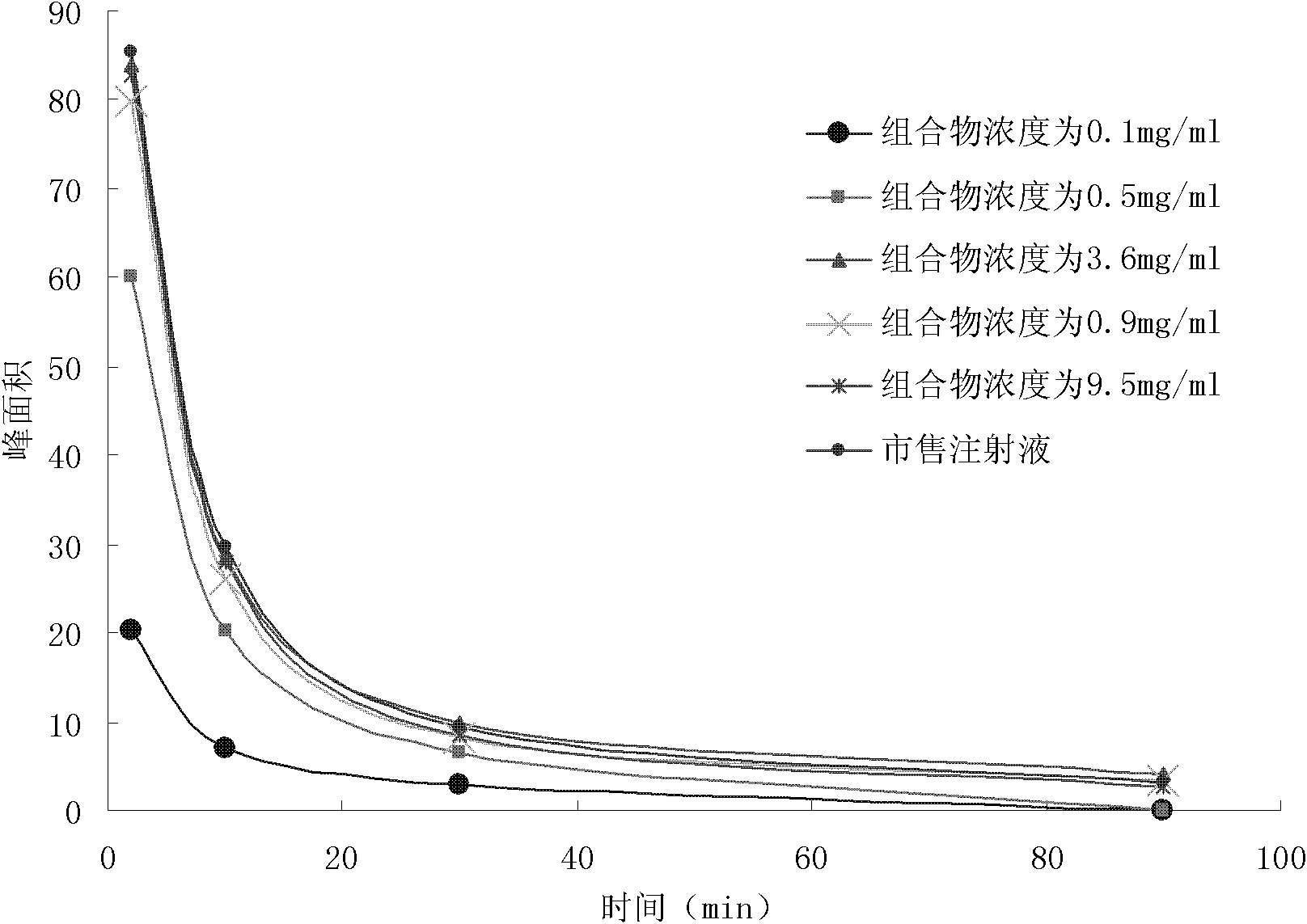
[0037] The present invention will now be described in detail in conjunction with the embodiments and drawings, but the implementation of the present invention is not limited thereto. Embodiment 1: A kind of composition of nimodipine injection
[0038] Preparation of Nimodipine Injection:
[0039] Weigh 4 grams of nimodipine, add 95 grams of polyethylene glycol 400, stir and dissolve at 60 ° C, then use polyethylene glycol 400 to make up to 100 ml; adjust the pH value to 4.50 with citric acid; add 0.2 grams Activated carbon was used for the needle, adsorbed at 60°C for 30 minutes, then filtered with a capsule filter, divided into 1ml / branch, sealed, and sterilized in a high-pressure steam sterilizer at 121°C for 15 minutes to obtain nimodipine Injection. Preparation of emulsion:
[0040] Weigh 100 grams of caprylic triglyceride for injection, 100 grams of soybean oil, heat in a water bath to 70°C, add 12 grams of egg yolk phospholipids for injection, cut to dissolve, stir an...
Embodiment 2
[0043] Embodiment 2: a kind of composition of nimodipine injection
[0044] Preparation of Nimodipine Injection:
[0045] Weigh 1 g of nimodipine, add it to 76 g of polyethylene glycol 400, stir and dissolve at 70°C, then use polyethylene glycol 400 to make the volume to 100 ml; add 0.01 g of activated carbon for needles, Adsorb at high temperature for 60 minutes, then filter with a microporous membrane, pack into 2ml / branch, seal, and sterilize with a circulating steam sterilizer at 100°C for 30 minutes to obtain nimodipine injection.
[0046] Preparation of emulsion:
[0047] Weigh 100 grams of caprylic triglyceride for injection, 100 grams of soybean oil, heat in a water bath to 80°C, add 12 grams of soybean lecithin for injection, shear to dissolve, stir and mix to obtain the oil phase; measure 700 grams of water for injection milliliter, add 22.5 grams of glycerin, stir to dissolve, and heat to 80°C to obtain the water phase; mix the oil phase and the water phase at a t...
Embodiment 3
[0050] Embodiment 3: A kind of composition of nimodipine injection
[0051] Preparation of Nimodipine Injection:
[0052] Weigh 5 grams of nimodipine, add it to 80 grams of polyethylene glycol 400, stir and dissolve at 80 ° C, then use polyethylene glycol 400 to make the volume to 100 ml; adjust the pH value to 3.5 with hydrochloric acid; add 0.1 g of Activated carbon for needles, adsorbed at 80°C for 45 minutes, then filtered with a microporous membrane, divided into 1ml / branch, sealed, and sterilized in a high-pressure steam sterilizer at 121°C for 15 minutes to obtain nimodipine injection liquid.
[0053] Preparation of emulsion:
[0054] Weigh 50 grams of caprylic triglyceride for injection, 50 grams of soybean oil, heat in a water bath to 65°C, add 30 grams of egg yolk phospholipids for injection, cut to dissolve, stir and mix to obtain the oil phase; measure 800 grams of water for injection milliliter, add 26 grams of glycerin, stir to dissolve, and heat to 65°C to ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com