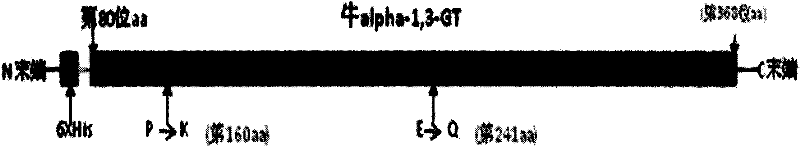Clone and expression of bovine recombinant alpha-1,3-galactoside transferase (GT)
A technology of galactoside and transferase, applied in the field of recombinant enzyme protein, can solve problems such as low activity, inability to apply clinically, and complicated procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
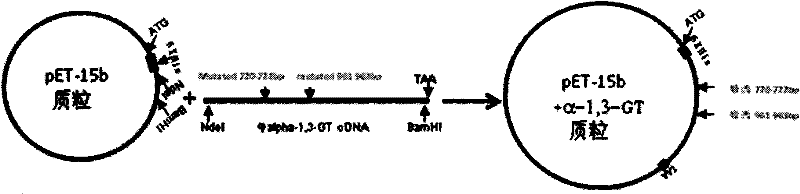
[0023] Example 1. Construction of expression recombinant bovine α-1,3-GT plasmid
[0024] The invention adopts the amino acid from the 80th to the 368th amino acid of the Inner Mongolia cow thymus GT protein, which is the smallest water-soluble part with GT activity. In order to further improve the water solubility and enzyme reactivity of recombinant GT, through the structural analysis of the enzyme protein, the two amino acids at the 160th and 241st positions have a greater impact on the activity and water solubility of the enzyme, so the present invention uses GT at the 160th Amend amino acid proline (P) to lysine (K); modify glutamic acid (E) at position 241 to glutamine (Q), see figure 1 .
[0025] First extract mRNA from bovine vascular endothelial cells, and amplify the bovine α-1,3-GT cDNA fragment through reverse transcription PCR (RT-PCR). The position is at 1104bp (namely at the 368th amino acid). The cDNA fragment amplified by reverse transcription PCR was clone...
Embodiment 2
[0026] Example 2. Purification of recombinant GTase protein
[0027] HMS174(DM3)E.coli containing pET-15b+α-1,3-GT plasmid was cultured to OD in LB medium 600 When = 0.7, add a final concentration of 1mM IPTG to induce the expression of the recombinant protein, continue to culture the bacteria at 35°C for 4 hours, centrifuge and collect the bacteria; freeze them on dry ice for 1.5 hours, then suspend them in the His-tag purification solution, and collect the supernatant by centrifugation , purified twice according to the His-tag purification column of Sigma Company, dialyzed three times in a cold storage at 4°C, and finally removed endotoxin with a kit. The purity of the expressed protein was determined by SDS-PAGE electrophoresis and high performance liquid phase, and quantified.
Embodiment 3
[0028] Embodiment 3. Activity test of recombinase protein
[0029] 3.1 Isotope test for α-1,3-GT enzyme activity
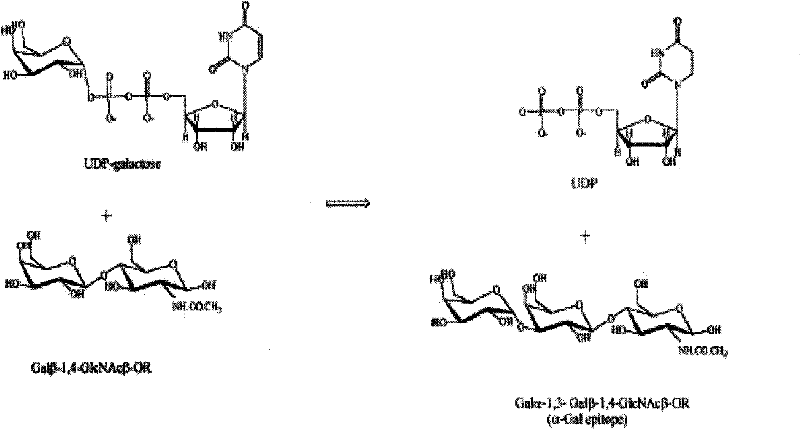
[0030] Test principle (see image 3): 100 μL containing 5 mM MnCl 2 , 5mM Tris-HCl (pH7.0), 50μM UDP-Gal (labeled isotope) and 10mM sugar acceptor Galβ-1, 4-GlcNAcβ-OR, added different concentrations of recombinant GT, reacted at 37°C for 15 minutes, added 20 μL ice water to stop the reaction. Add 0.05ml of the reaction solution into a liquid scintillation vial of AG1-X8 anion exchange resin (Bio-Rad), and measure the isotope after repeated washing.
[0031] Quantitative standard of enzyme activity: 1 unit of enzyme = transfer of 1 μmol Gal from UDP-Gal to Galβ-1,4-GlcNAcβ-OR sugar acceptor within 15 minutes at 37°C.
[0032] Test Results:
[0033] A. The expression amount of enzyme protein of the present invention: >10 mg of enzyme protein can be produced per liter of LB culture solution; enzyme activity: 15 units / mg. Electrophoresis confirmed that the mol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com