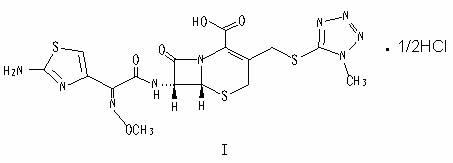
Preparation method of cefmenoxime hydrochloride
A technology of cefmenoxime hydrochloride and cefmenoxime, which is applied in the field of preparation of cefmenoxime hydrochloride to achieve the effects of less environmental pressure, reducing degradable impurities and shortening production time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
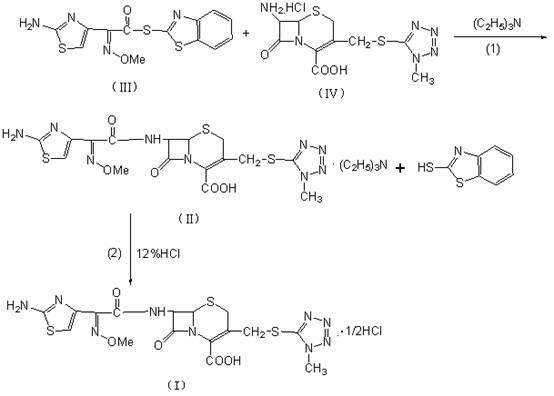
[0031] Step (1): Take 25kg of 7-ATCA·HCl and 30kg of AE active ester, add them to the mixture of 250L of dichloromethane and 25L of ethanol, add 24L of triethylamine dropwise within 60 minutes, and control the reaction temperature at 25°C , and the reaction was stirred for 1.5 hours. Add 200L of water for hydrolysis, extract the organic phase with 50ml of water twice, combine the water phase, add 25 kg of γ-alumina to the water phase, stir at 25°C, stir for 2 hours to decolorize, and filter through a press filter and a sterilizing filter element Into a crystallization tank in a sterile room, wash gamma-alumina with 200L of water, and combine the water phases to obtain an aqueous solution of cefmenoxime triethylamine salt.
[0032] Step (2): Control the temperature of the aqueous solution of cefmenoxime triethylamine salt at 5°C, adjust the pH to 2.2 with 12wt% hydrochloric acid, and precipitate a white solid, stir and grow crystals for 3 hours, filter, and wash the filter cake...
Embodiment 2
[0034] Step (1): Take 25kg of 7-ATCA·HCl and 30kg of AE active ester, add them to the mixture of 250L of dichloromethane and 25L of acetone, add 24L of triethylamine dropwise within 60 minutes, and control the reaction temperature at 25°C , and stirred for 2 hours. Add 200L of water for hydrolysis, extract the organic phase twice with 50L of water, combine the water phase, add 50 kg of γ-alumina to the water phase, stir at 25°C, stir for 2 hours to decolorize, and filter through a press filter and a sterilizing filter element Into a crystallization tank in a sterile room, wash gamma-alumina with 200L of water, and combine the water phases to obtain an aqueous solution of cefmenoxime triethylamine salt.
[0035] Step (2): Control the temperature of the aqueous solution of cefmenoxime triethylamine salt at 5°C, adjust the pH to 2.2 with 12wt% hydrochloric acid, and precipitate a white solid, stir and grow crystals for 2 hours, filter, and wash the filter cake with methanol. Dry...
Embodiment 3
[0037]Step (1): Take 25 kg of 7-ATCA·HCl, IV) and 30 kg of AE active ester, add them to the mixture of 250 L of ethyl acetate and 25 L of DMAC, add 24 L of triethylamine dropwise within 60 minutes, and control the reaction temperature at -5°C, stirred and reacted for 2 hours. Add 200L of water for hydrolysis, extract the organic phase twice with 50L of water, combine the water phase, add 50 kg of γ-alumina to the water phase, stir at 25°C, stir for 2 hours to decolorize, and filter through a press filter and a sterilizing filter element Into a crystallization tank in a sterile room, wash gamma-alumina with 200L of water, and combine the water phases to obtain an aqueous solution of cefmenoxime triethylamine salt.
[0038] Step (2): Control the temperature of the aqueous solution of cefmenoxime triethylamine salt at 10°C, adjust the pH to 2.2 with 12wt% hydrochloric acid, and precipitate a white solid, stir and grow crystals for 2 hours, filter, and wash the filter cake with me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com