Method for separating and purifying high-purity activated clotting seventh factors from cell culture solution or plasma components
A cell culture, separation and purification technology, applied in the field of separation and purification of high-purity activated blood coagulation factor seven, can solve problems such as adverse reactions, achieve good safety, simple and efficient thrombin activation process, and improve comprehensive utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The preparation of embodiment 1 affinity chromatography medium
[0053] Affinity chromatography medium: CNBr-activated Sepharose 4Fast Flow or CNBr-activated Sepharose 4B product from GE Healthcare.
[0054] Preparation of seven-factor monoclonal antibodies:
[0055] Construct hybridoma cells according to conventional methods, and extract RNA from hybridoma cells that can produce anti-activated blood coagulation factor VII, convert RNA into single-stranded DNA in reverse transcription reaction, and then amplify with two pairs of designed primers respectively. The light chain and heavy chain variable region sequences were increased, and the obtained products were respectively cloned into pMD18-T vector. Sequence the obtained light chain and heavy chain sequences, and design the whole gene sequences of the light chain and heavy chain according to the sequencing results:
[0056] Light chain DNA sequence: (SEQ ID NO: 3)
[0057] ATGGGATGGTCCTGCATCATCCTGTTCCTGGTGGCAACTGC...
Embodiment 2
[0068] Example 2 Component II+III affinity chromatography to separate seven factors
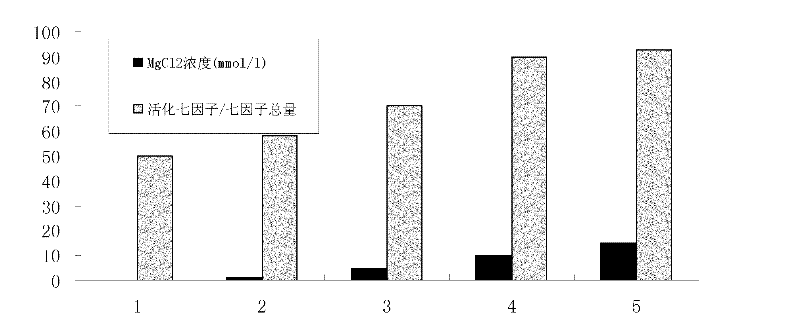
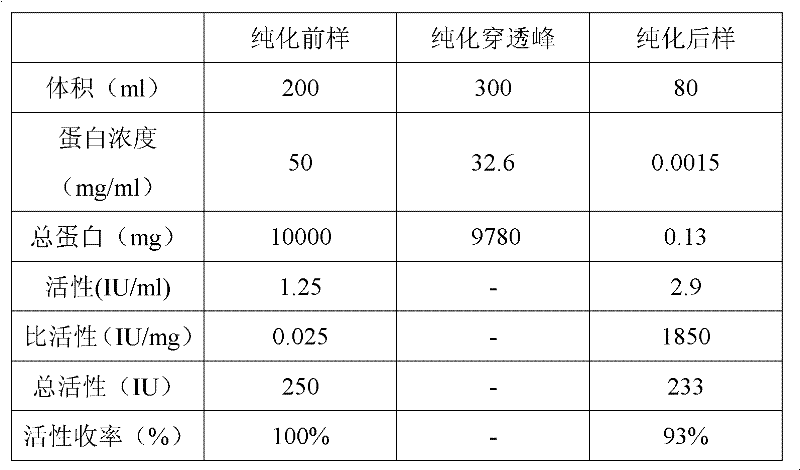
[0069] 100ml of plasma fraction II+III sample with a protein content of 10% was added to 100ml of pH7.4 sodium dihydrogen phosphate / disodium hydrogen phosphate buffer solution to dilute evenly, and used as the sample before affinity chromatography purification. Seven-factor antibody affinity chromatography column 50ml equilibrated with pH 7.4 sodium dihydrogen phosphate / disodium hydrogen phosphate buffer solution for 5 column volumes, flow rate 5ml / min, loading 200ml of component II+III dilution, control flow rate 3ml / min, collecting 300ml of the breakthrough peak. After loading the sample, rinse with sodium dihydrogen phosphate / disodium hydrogen phosphate buffer solution to the baseline. Use pH 2.5-5.0 glycine / hydrochloric acid buffer solution to elute the seven factors (preferably pH 2.5 glycine / hydrochloric acid buffer solution to elute the seven factors), the elution flow rate is 5ml / mi...
Embodiment 3
[0072] Example 3 Separation of Seven Factors by Affinity Chromatography Using Seven Factors Cell Culture Fluid as Raw Material
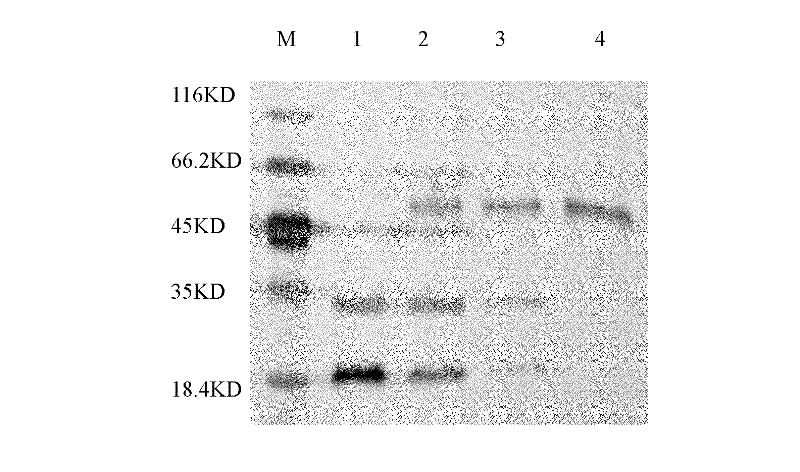
[0073] Suspension culture of seven-factor cells in serum-free medium in a bioreactor, when the cell density reaches 1X10 7 Cells / ml, when the concentration of seven factors reaches 10 mg / l, collect 20 liters of supernatant samples of the culture medium, use pH 7.4 sodium dihydrogen phosphate / disodium hydrogen phosphate buffer solution for ultrafiltration and concentration to 2000 ml, and use it as affinity chromatography Samples before purification. 50ml of seven-factor antibody affinity chromatography column was equilibrated with 5 column volumes of sodium dihydrogen phosphate / disodium hydrogen phosphate buffer solution with pH 7.4, the equilibrium flow rate was 5ml / min, 2000ml of plasma sample was loaded, 2150ml of breakthrough peak was collected, and The sample flow rate is 10ml / min. After loading the sample, rinse with sodium dihydrogen phospha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com