Vinpocetine injection and preparation method thereof
A technology of vinpocetine and injection, applied in the field of preparation of vinpocetine injection, which can solve the problems of strong vascular irritation, poor safety, large dosage, etc., and achieve high safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
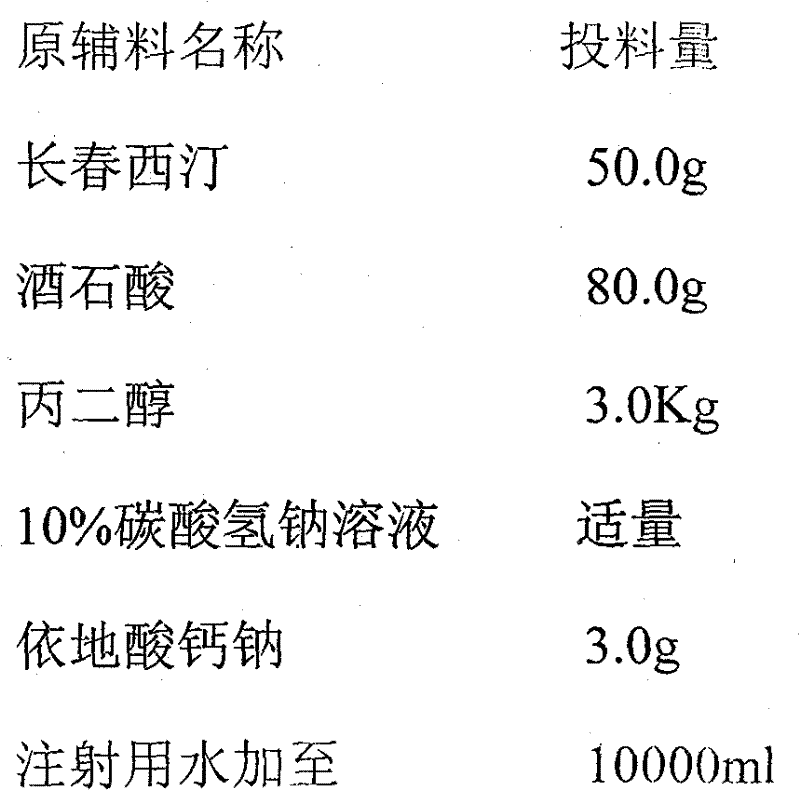
[0023] prescription:
[0024]
[0025] Preparation method:
[0026] As shown in Figure 1, take 50% water for injection, add calcium sodium edetate, stir to dissolve; add vinpocetine, add tartaric acid, stir to completely dissolve the raw materials; add propylene glycol, stir evenly; slowly add 10% sodium bicarbonate Solution, stir while adding, adjust the pH value to 4.0~6.0; add water for injection to the full amount, stir evenly, add 0.02% activated carbon, let stand for 20 minutes, filter and decarbonize; detect the properties of the liquid (colorless clear liquid), After the pH value (4.0-6.0) and content (97%-103%) are qualified, filter (0.45 μm, 0.22 μm), fill with nitrogen gas, sterilize by autoclaving at 115°C for 30 minutes, inspect by light, and pack.
example 2
[0028] prescription:
[0029]
[0030]
[0031] Preparation method:
[0032] Take 50% water for injection, add calcium sodium edetate, stir to dissolve; add vinpocetine, add citric acid, stir to completely dissolve the raw materials; add propylene glycol, stir evenly; slowly add 10% sodium bicarbonate solution, while Stir while adding, adjust the pH value to 4.0~6.0; add water for injection to the full amount, stir evenly, add 0.02% activated carbon, let stand for 20 minutes, filter and decarbonize; detect the properties of the medicinal solution (colorless clear liquid), pH value ( 4.0-6.0), the content (97%-103%) is qualified, filtered (0.45 μm, 0.22 μm), filled with nitrogen gas, sterilized by autoclaving at 115° C. for 30 minutes, inspected by light, and packaged.
example 3
[0034] prescription:
[0035]
[0036] Preparation method:
[0037] Take 50% water for injection, add calcium sodium edetate, stir to dissolve; add vinpocetine, add tartaric acid, stir to completely dissolve the raw materials; add glycerin, stir evenly; slowly add 10% sodium bicarbonate solution, add as you go Stir and adjust the pH value to 4.0 to 6.0; add water for injection to the full amount, stir evenly, add 0.02% activated carbon, let stand for 20 minutes, filter and decarbonize; detect the properties of the medicinal solution (colorless clear liquid), pH value (4.0~ 6.0) and the content (97%-103%) are qualified, filtered (0.45 μm, 0.22 μm), filled with nitrogen, filled with nitrogen, sterilized by autoclaving at 115°C for 30 minutes, inspected by light, and packaged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com