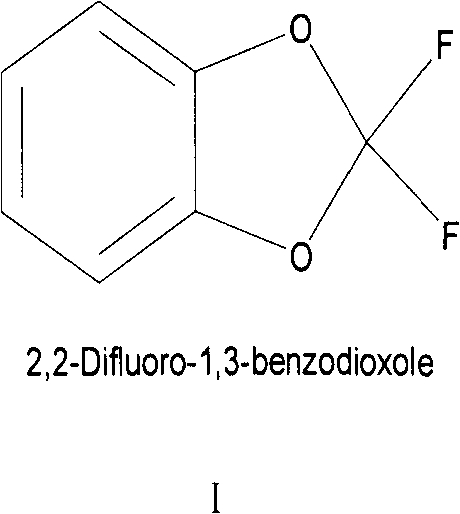
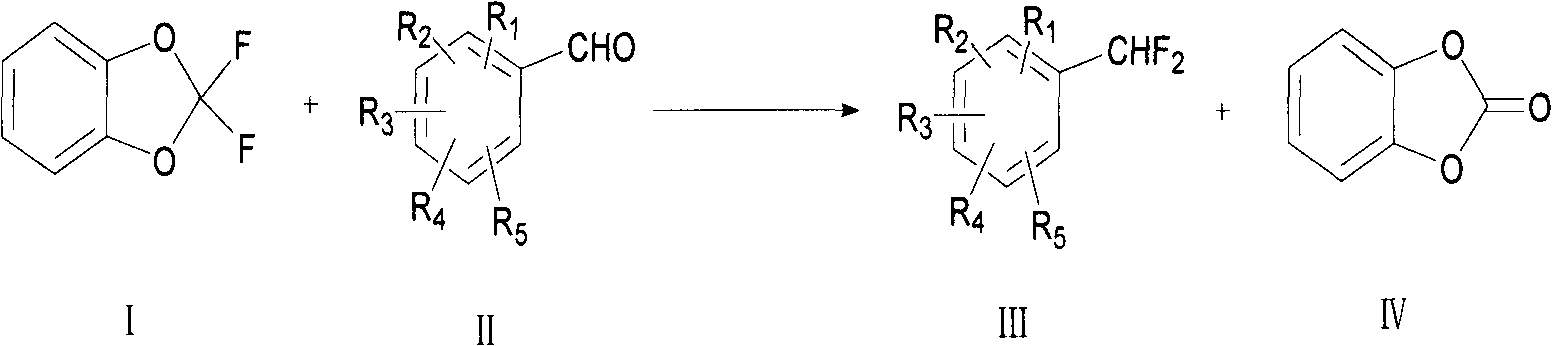
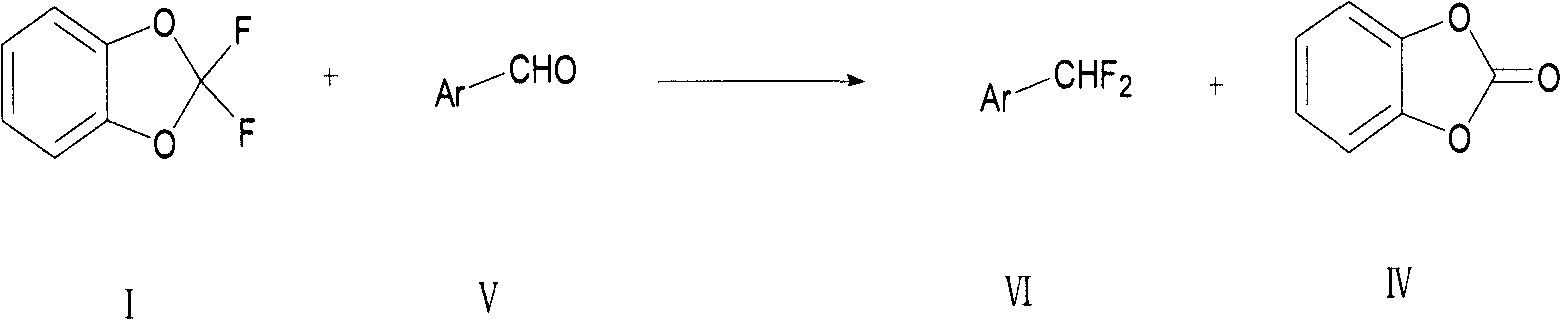
Preparation method for fluoride aromatic organic compound
An organic compound, difluoromethyl aromatic technology, applied in the field of chemical synthesis routes, can solve problems such as expensive, unstable pure products, lack of fluorine, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] As for the production method and fluorinating agent of the present invention, it is preferable to use a catalyst selected from acid, Lewis acid or water in addition to 2,2-difluoro-1,3-benzodioxol. More preferably, an acid is used as a catalyst for this reaction.
[0039] Specific examples of the catalyst include sulfuric acid, nitric acid, phosphoric acid, polyphosphoric acid, hydrogen fluoride, hydrofluoric acid, hydrochloric acid, hydrogen bromide, hydrogen iodide, hypochlorous acid, chlorous acid, chloric acid, perchloric acid, perbromic acid , hydrogen halides such as periodic acid, or hydrohalic acid, hypohalous acid, halous acid, halogen acid and perhalogen acid;
[0040]Fluorosulfonic acid, chlorosulfonic acid, methanesulfonic acid, ethanesulfonic acid, trifluoromethanesulfonic acid, difluoromethanesulfonic acid, trichloromethanesulfonic acid, perfluorobutylsulfonic acid, perfluorooctanesulfonic acid , benzenesulfonic acid, toluenesulfonic acid, nitrobenzenesul...
Embodiment 1
[0055] Preparation of 2,2-difluoro-1,3-benzodioxol:
[0056] step one:
[0057] Dissolve piperonylcycline (244g, 2mol, 1.0eq) and AIBN (2.00g) in 500ml benzotrifluoride, add 100ml benzotrifluoride to a four-neck flask, heat to reflux, add piperonylcycline / AIBN / trifluorotoluene dropwise Chlorine gas was introduced into the toluene solution at the same time, and the passage was completed in 8 hours, and the stirring was continued for 0.5 hours. Then the reaction solution was desolvated under reduced pressure, and then 2,2-dichloro-1,3-benzodioxol was distilled off under reduced pressure to obtain 363 g with a yield of 95%.
[0058] Step two:
[0059] Put 2,2-dichloro-1,3-benzodioxol (363g, 1.9mol) in a 1000ml polytetrafluoroethylene reaction bottle, pass through 84g of hydrogen fluoride gas at 40°C, and pour it into water after passing through, Separate the liquid, wash the organic phase with water, add anhydrous magnesium sulfate to dry, filter, and rectify under reduced pre...
Embodiment 1~20
[0064] Add m-fluorobenzaldehyde A (2.00g, 16mmol, 1.0eq), 2,2-difluoro-1,3-benzodioxol B (3.06g, 19mmol, 1.2eq) into a 50ml polytetrafluoroethylene bottle Add 10ml of a certain solvent or no solvent, add a catalyst, raise the temperature to a certain level, and react for several hours. Stop the reaction, add 10ml of dichloromethane, wash the organic phase with 10ml of water after complete dissolution, wash the organic phase with 10ml of saturated sodium bicarbonate solution after liquid separation, and filter after liquid separation to obtain the organic phase. The product was isolated and purified using column chromatography. The product was analyzed by NMR and MS, and the reaction conversion rate was calculated by gas chromatography column.
[0065] React according to a similar method, specifically as shown in Table 1 below:
[0066] Table 1: Reaction results of different substrates
[0067]
[0068]
[0069]
[0070] The spectral data of the compounds obtained i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com