Lansoprazole composition for injection
A technology for lansoprazole and a composition, applied in the field of pharmaceutical preparation, can solve problems such as unsolved technical defects, and achieve the effects of improving quality level, reducing production cost and preventing reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
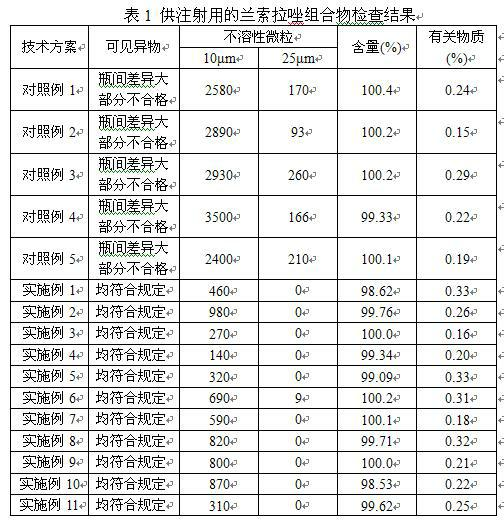
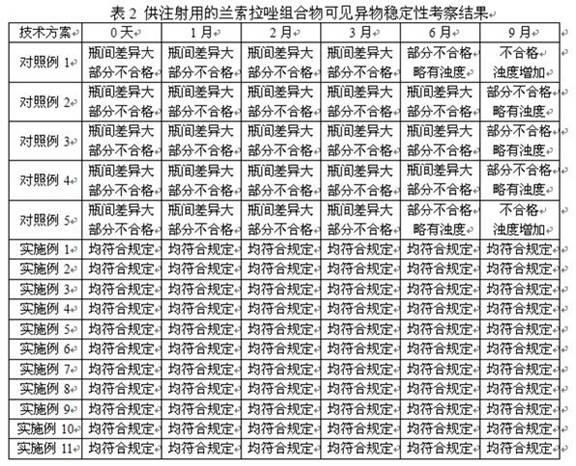
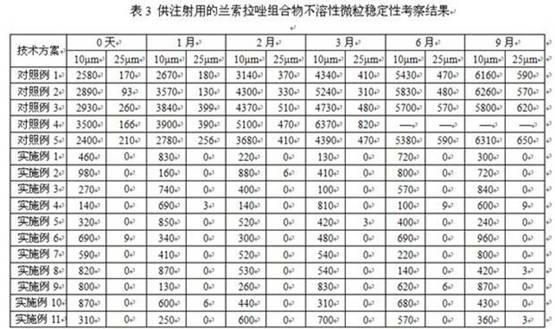
Image
Examples
Embodiment 1
[0070] Embodiment 1 prepares the lansoprazole composition for injection
[0071] prescription:
[0072] Lansoprazole 300g
[0073] EDTA 6g
[0074] Add water for injection to 20Kg
[0075] 1) Preparation of liquid medicine: Weigh lansoprazole and disodium edetate into a preparation tank, add water for injection, stir to mix evenly, add sodium hydroxide to dissolve and adjust the pH value of the solution to 11.5;
[0076] 2) Rubber stopper treatment:
[0077] a. Take disodium edetate, add water for injection according to the weight ratio of disodium edetate and water for injection 1:1000, adjust the pH value to 7.0 with sodium hydroxide after stirring and dissolving;
[0078] b. Take the rubber plug, add solution a according to the weight ratio of rubber plug and solution a 1:10, heat to 45°C and keep it warm for 30 minutes;
[0079] c. Take the rubber stopper that has been treated in the process of b, rinse it with water for injection filtered through a 0.22 μm filter for...
Embodiment 2
[0082] Embodiment 2 prepares the lansoprazole composition for injection
[0083] prescription:
[0084] Lansoprazole 300g
[0085] EDTA 9g
[0086] Add water for injection to 20Kg
[0087] 1) Preparation of liquid medicine: Weigh lansoprazole and disodium edetate into a preparation tank, add water for injection, stir to mix evenly, add sodium hydroxide to dissolve and adjust the pH value of the solution to 11.7;
[0088] 2) Rubber stopper treatment:
[0089] a. Get disodium edetate, add water for injection according to the weight ratio of disodium edetate and water for injection 1:1000, adjust the pH value to 6.0 with sodium hydroxide after stirring and dissolving;
[0090] b. Take the rubber stopper, add solution a according to the weight ratio of the stopper and solution a of 1:10, heat to 41°C and keep it warm for 30 minutes;
[0091] c. Take the rubber stopper that has been treated in the process of b, rinse it with water for injection filtered through a 0.22 μm filte...
Embodiment 3
[0094] Embodiment 3 prepares the lansoprazole composition for injection
[0095] prescription:
[0096] Lansoprazole 300g
[0097] EDTA 12g
[0098] Add water for injection to 20Kg
[0099] 1) Preparation of liquid medicine: Weigh lansoprazole and disodium edetate into a preparation tank, add water for injection, stir to mix evenly, add sodium hydroxide to dissolve and adjust the pH value of the solution to 11.9;
[0100] 2) Rubber stopper treatment:
[0101] a. Get disodium edetate, add water for injection according to the weight ratio of disodium edetate and water for injection 1:1000, adjust the pH value to 6.5 with sodium hydroxide after stirring and dissolving;
[0102] b. Take the rubber stopper, add solution a according to the weight ratio of the stopper and solution a of 1:10, heat to 47°C and keep it warm for 30 minutes;
[0103] c. Take the rubber stopper that has been treated in the process of b, rinse it with water for injection filtered through a 0.22 μm filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com