Separation and purification method for liposome containing encapsulated material
A purification method and liposome technology, applied in the field of biomedicine, can solve the problems of time-consuming and labor-intensive effects, consumption of phospholipid raw materials, long time, etc., and achieve the effects of simple operation, efficient recovery and wide application range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
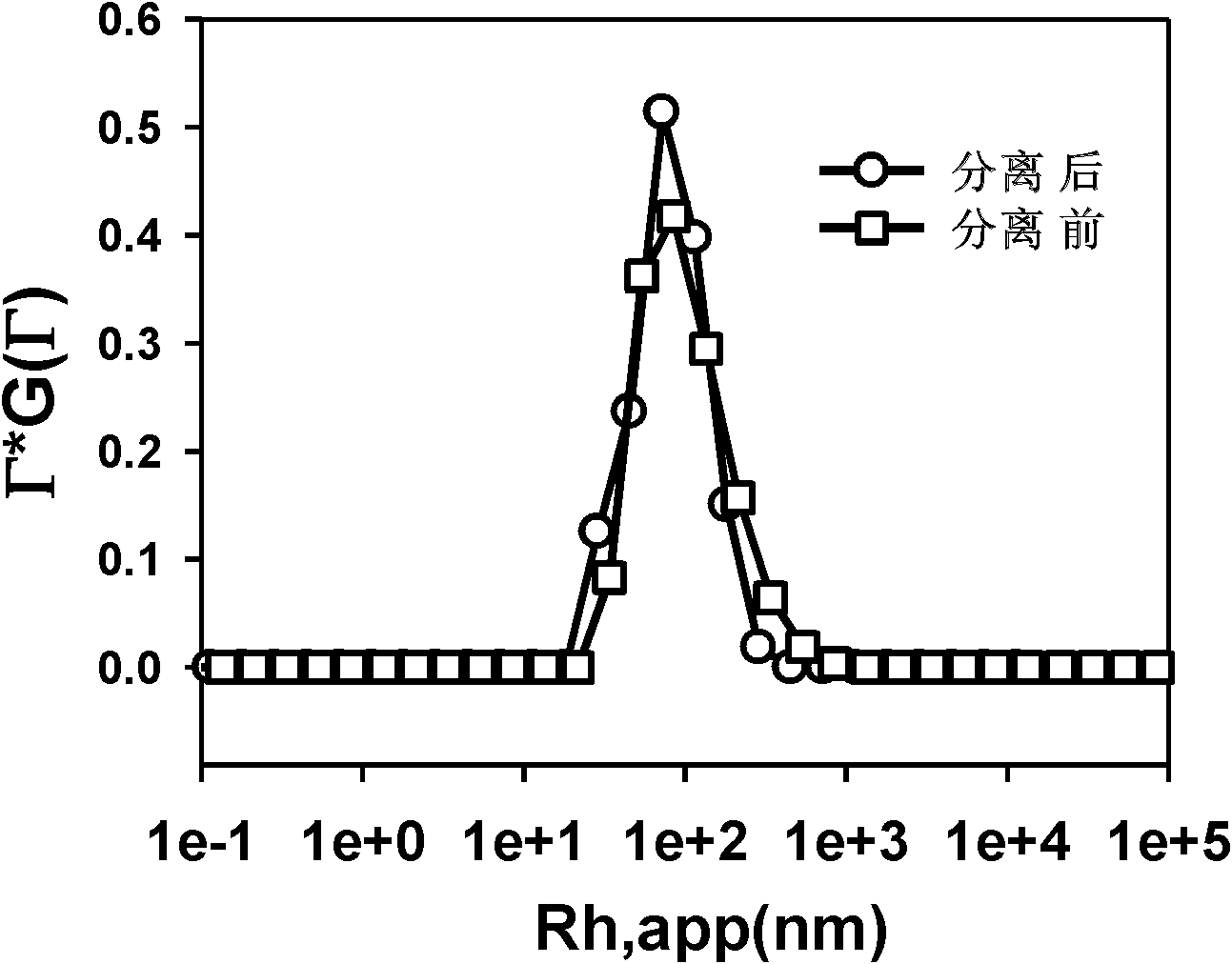
Image
Examples
Embodiment 1
[0019] Embodiment 1. Taking the encapsulated substance as a negatively charged small molecule (such as the commonly used model fluorescent molecule calcein, and the commonly used analgesic drug ibuprofen) as an example
[0020] 1. Preparation of electrophoresis buffer
[0021] Fully dissolve Tris (10.78g), boric acid (5.50g), and EDTA (0.74g) in 100mL of water, and filter through a filter membrane with a pore size of 0.45um to obtain a stock solution of the electrophoresis buffer. Diluted 20 times for use.
[0022] 2. Preparation of drug model solution
[0023] Here, calcein is selected as a model drug molecule to test the separation effect of this method. The molecular structure of calcein is It is not the actual drug used and has no medicinal effect. But because of the fluorescence, it is convenient for characterization. Calcein (311 mg, 0.5 mmol) was added to 5 mL of electrophoresis buffer, stirred evenly until the calcein was completely dissolved, and then 4 mol / L Na...
Embodiment 2
[0034] Embodiment 2, taking the encapsulated substance as a positively charged small molecule (such as the commonly used anticancer drug doxorubicin) as an example:
[0035] The molecular structural formula of doxorubicin is:
[0036] 1. Preparation of liposomes encapsulated with doxorubicin
[0037] The chloroform solution of distearoylphosphatidylcholine and cholesterol was taken, and the chloroform solution was removed by rotary evaporation to obtain a phospholipid film. The liposomes were prepared by freezing and thawing or extrusion by hydration with a citrate buffer solution with a pH of 4.0. The final concentration of phospholipids was 30 mM, and then the liposomes were eluted with a gel column saturated with hydroxyethylpiperazineethanesulfonic acid buffer solution to form a pH gradient inside and outside the liposomes. Dissolve doxorubicin in normal saline at a concentration of 2 mg / mL. The liposome and doxorubicin solution were mixed at a volume ratio of 5:1, an...
Embodiment 3
[0045] Embodiment 3, taking encapsulated substances as charged macromolecules (such as DNA, siRNA, etc.) as an example:
[0046] 1, the preparation method of the liposome that has encapsulated DNA
[0047] Firstly, liposomes composed of egg yolk phosphatidylcholine were prepared at a concentration of 80 mM. Take 250 μL of liposome solution and blend with 100 μL of protist DNA at a concentration of 1 mg / mL. Add ethanol and CaCl to the above mixed solution 2 Aqueous solution, vortexed for 30s and then dialyzed for 24 hours.
[0048] 2. Gel electrophoresis separation of liposomes encapsulating DNA
[0049] Prepare an agarose gel with a concentration of 0.5%, and you can choose spot staining or bubble staining to determine the separation of DNA. Acrylamide gel can also be used as the separation medium, the voltage is 9V / cm, electrophoresis is carried out for 2 hours, and the unencapsulated DNA can be separated from the liposome. Liposomes and free DNA molecules can be recover...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com