Preparation method of triethyl citrate
A technology of triethyl citrate and citric acid, which is applied in the field of preparation of triethyl citrate, can solve the problems of difficult reaction end point, many reaction side reactions, and dimness, so as to reduce side reactions, prevent insufficient reaction, reduce Harmful effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
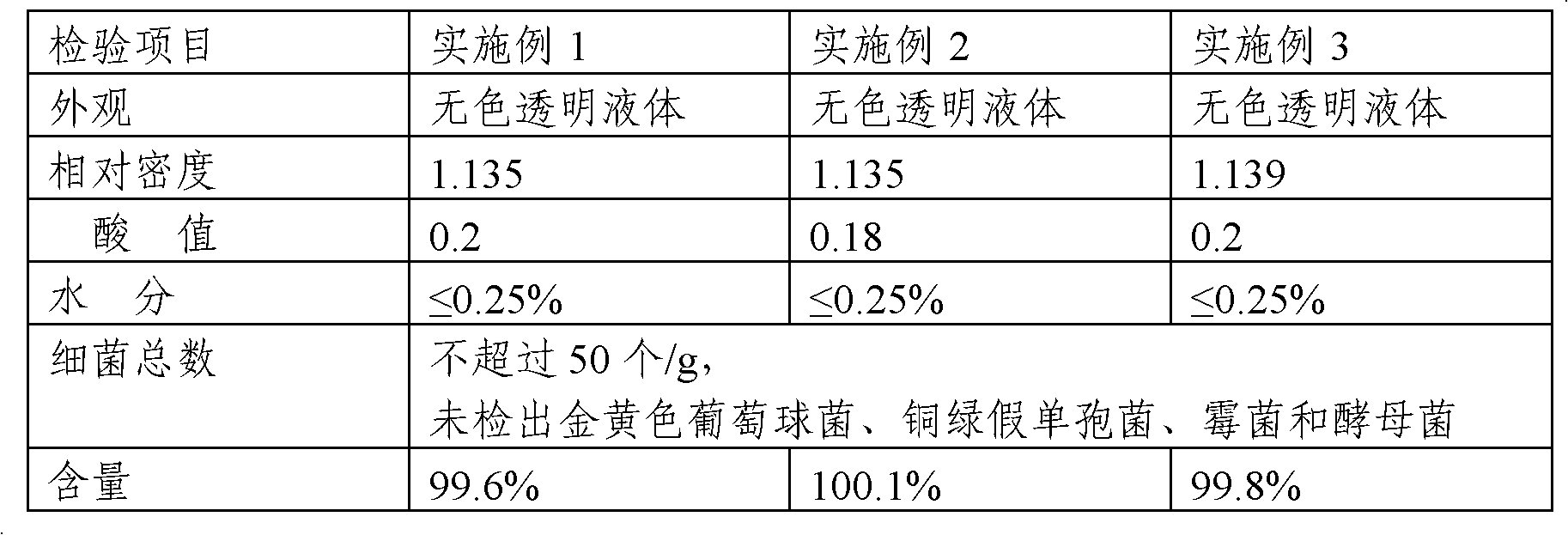
Embodiment 1
[0026] 1. Preparation of crude triethyl citrate: In a 2500ml three-necked bottle, install a thermometer, condenser, and mechanical stirring. Add 1200g of absolute ethanol (or recovered ethanol: more than 95%), start stirring and preheat to 50°C, add 800g of citric acid, 6ml of concentrated sulfuric acid and slowly heat to reflux, and collect the distillate as the next batch of feed. Control the distillation speed to about 20ml / hour (the addition of absolute ethanol in time is the same as the amount of distillate) to react for 20 hours, and start to detect the end point.
[0027] Use a pipette to pipette 1ml of the reaction feed solution into a small beaker and add 3-5 drops of phenolphthalein indicator. Titrate with 1% sodium hydroxide titration until the feed liquid turns red, record the amount of sodium hydroxide titration, when the amount of 1% sodium hydroxide titration is less than 5ml, it can be regarded as complete reaction, when the acid value is less than 5, it can be...
Embodiment 2
[0030] 1. Preparation of crude triethyl citrate: In a 2500ml three-necked bottle, install a thermometer, condenser, and mechanical stirring. Add 1000g of absolute ethanol (or recovered ethanol: more than 95%), start stirring and preheat to 60°C, add 800g of citric acid, 6g of sodium benzenesulfonate and slowly heat to reflux to collect the distillate as the next batch of feed. Control the distillation rate to about 28ml / hour (the addition of absolute ethanol in time is the same as the amount of distillate) to react for 22 hours, and start to detect the end point.
[0031] Use a pipette to pipette 1ml of the reaction feed solution into a small beaker and add 3-5 drops of phenolphthalein indicator. Titrate with 1% sodium hydroxide titration solution until the feed liquid turns red, record the sodium hydroxide titration solution consumption, treat that the reaction is complete when the 1% sodium hydroxide titration solution consumption is less than 5ml, and distill off excessive ...
Embodiment 3
[0034] 1. Preparation of crude triethyl citrate: In a 200°C reaction kettle, install a thermometer, condenser, and mechanical stirring. Add 120kg of absolute ethanol (or recovered ethanol: more than 95%), start stirring and preheat to 50°C, add 80kg of citric acid, 600ml of concentrated sulfuric acid and slowly heat to reflux, and collect the distillate as the next batch of feed. Control the distillation speed to about 20 liters / hour (the addition of absolute ethanol in time is the same as the amount of distillate) and react for 20 hours, and start to detect the end point.
[0035] Use a pipette to pipette 1ml of the reaction feed solution into a small beaker and add 3-5 drops of phenolphthalein indicator. Titrate with 1% sodium hydroxide titration until the feed liquid turns red, record the amount of sodium hydroxide titration, when the amount of 1% sodium hydroxide titration is less than 5ml, it can be regarded as complete reaction, when the acid value is less than 5, it can...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com