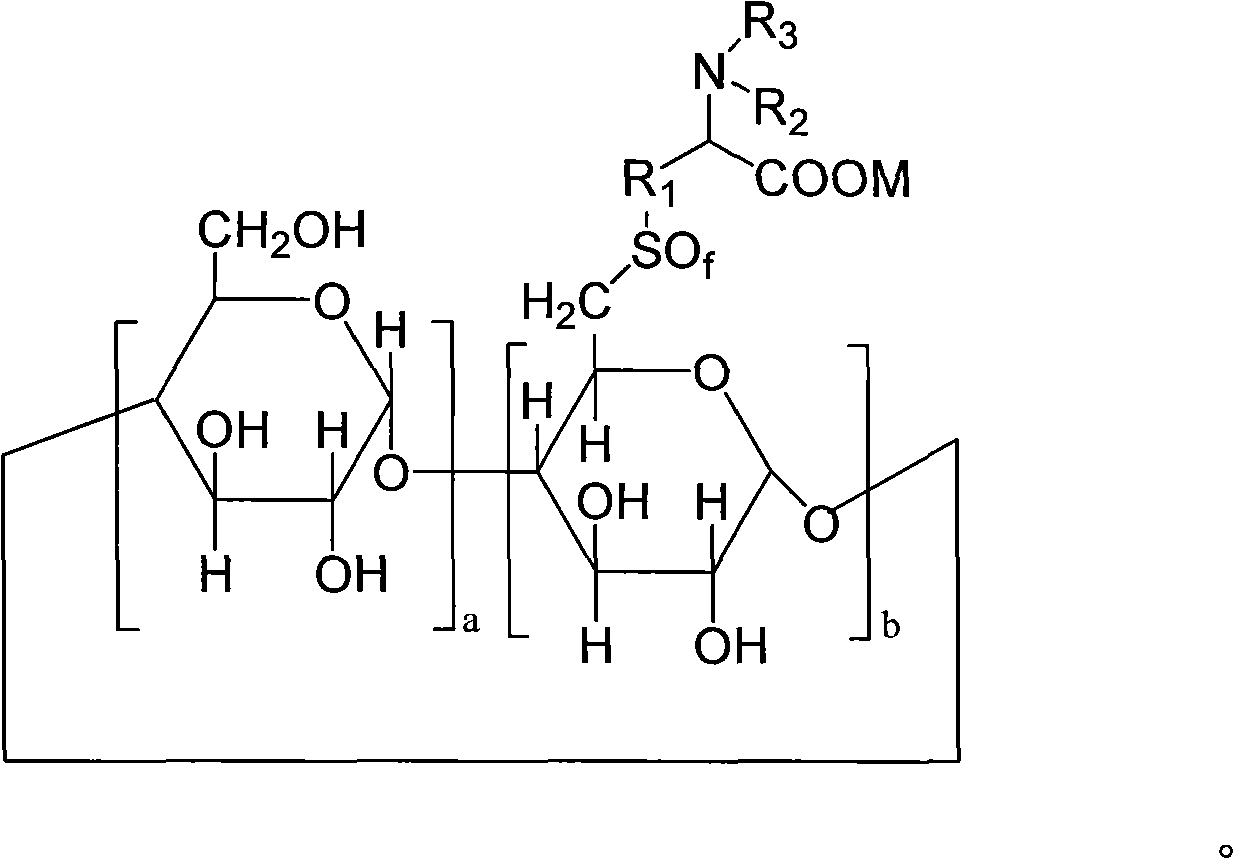
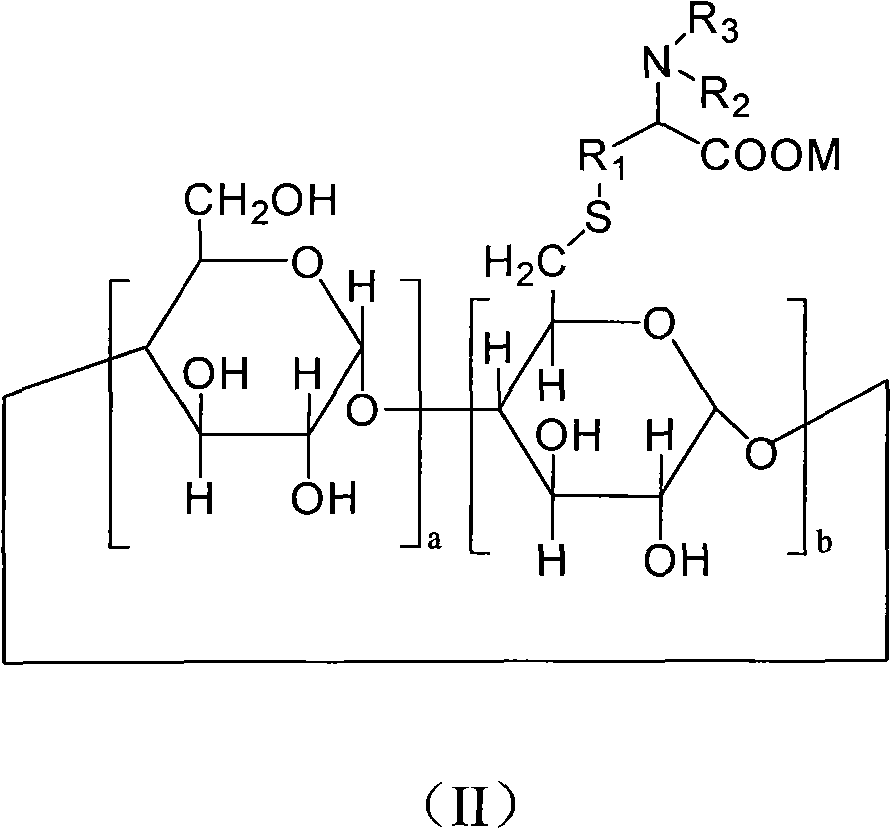
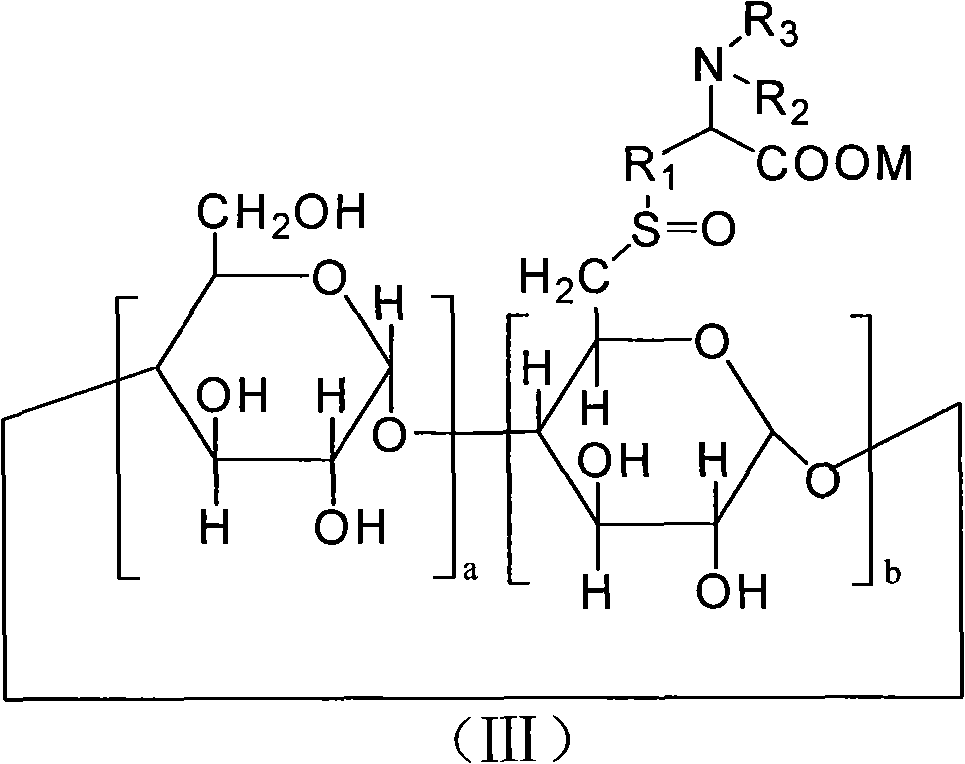
6-deoxy alpha-amino acid derivative cyclodextrin, preparation and application thereof
A derivative and amino acid technology, which is applied in the field of 6-deoxy α-amino acid derivative cyclodextrin and its preparation and application, can solve the problems of poor antagonistic effect and exclusion, and achieve stable preparation process, high yield and strong Effects of Binding and Selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049]
[0050]A solution of L-cysteine (1.5g, 8.54mmol) in water (5ml) was added to the reaction flask, and mixed with 1M sodium bicarbonate (25.62ml) and benzyl chloroformate 1.32ml (9.41mmol). The reaction was stirred at 0°C for 4 hours, then cooled to room temperature and extracted with ether to remove excess benzyl chloroformate. The aqueous phase was acidified to PH=3 by adding hydrochloric acid, extracted with ethyl acetate, dried, and the solvent was removed to obtain the product (CD-1) with a yield of 93%.
[0051]
[0052] Weigh dry CD-16g (0.024mol), measure dehydrated 60ml DMF, add to a dry three-necked flask, stir until fully dissolved and colorless solution. The temperature of the reaction solution was lowered to -20°C under a constant temperature cooling bath, and 2.35 g (0.059 mol) of sodium hydride (60%) was slowly added in batches, protected by argon, mechanically stirred, and the temperature was controlled below -5°C. Continue to stir until no bubbl...
Embodiment 27
[0139] Dutch guinea pigs were injected with atropine 30 minutes before the operation, and after intraperitoneal anesthesia with pentobarbital sodium, the guinea pigs were fixed on the mouse board, connected to a small animal ventilator after endotracheal intubation, and the stimulation electrodes of the TOF-Watch SX muscle relaxation monitor were respectively It was connected under the skin of the left femur and posterior tibia of the guinea pig, and the left tibia was fixed on a small platform with self-made tools, and the left hind paw could move freely. The sensor was fixed on the palm of the left hind paw of the guinea pig, and the skin temperature probe was fixed on the palm of the left forepaw. Adjust 4 serial stimulations (TOF, frequency 2 Hz, wave width 0.2 ms, interval between strings 15 s), TOF stimulation voltage 5 mA; adjust the sensitivity so that T1 is stable for more than 5 min, and inject rocuronium bromide intravenously , 50mg / 5ml) 0.16mg / kg (2 times ED90 dose...
Embodiment 28
[0148] Male mice were given the compound of the present invention by tail vein injection, and the toxic reaction was observed. See Table 2 for the results.
[0149] Table 2
[0150] group
[0151] CD-30
[0152] According to literature reports, the maximum immortal dose of CN1402737's marketed product Bridion mice is 2000mg / kg, given the compounds of the present invention CD-8, CD-10, CD-19, CD-23, CD-28, CD-30 or CD-32 When the dose was 4000mg / kg, the mice were still normal, indicating that the safe dose of the compound of the present invention was doubled, and the drug safety was high.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com