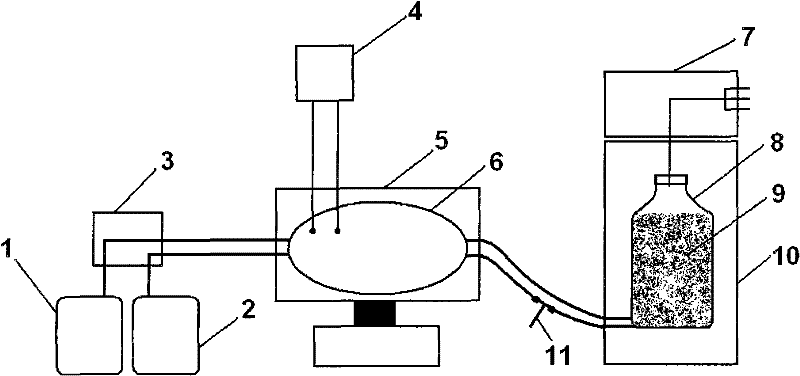
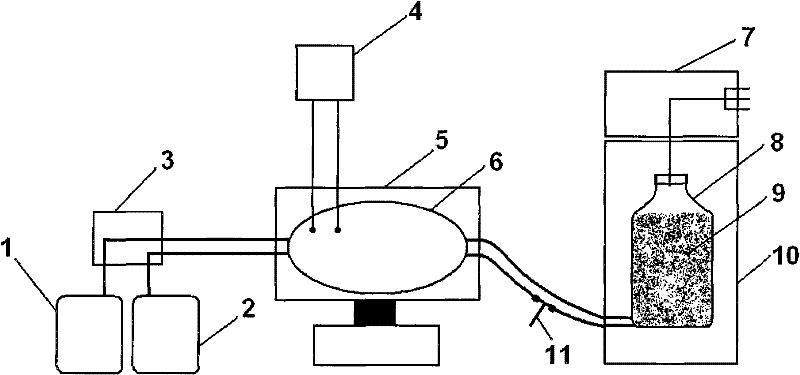
Method for producing influenza viruses in large scale by using bioreactor
A bioreactor, influenza virus technology, applied in the fields of biochemical equipment and methods, microorganisms, viruses/phages, etc., can solve the problems of not being able to adjust in real time, unable to ensure the optimal culture state of cells, etc., to achieve no contamination by exogenous factors, Real-time control of cell culture conditions and low contamination rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Influenza virus: H9N2 subtype influenza virus HN strain (see literature: Avian influenza virus (H9N2 subtype, HL strain) and avian influenza virus (H9 subtype) epidemic strain antigen correlation and cross-immunity research in some provinces in China, 2007 Proceedings of the Annual Meeting of the Society of Animal Husbandry and Veterinary Medicine, 2007, 35-38), the applicant holds the virus strain and is willing to distribute it to the public within 20 years from the date of application according to the relevant provisions of the Patent Law.
[0063] Bioreactor and carrier: Tidecell tidal bioreactor (carrier tank with a volume of 20 L) and "BioNOC II" carrier were purchased from Taiwan CESCO Bioengineering Company.
[0064] Passage cell line: MDCK (NBL-2) (this cell line is deposited with ATCC under the accession number CCL-34, see http: / / www.atcc.org for details).
[0065] Serum-free cell culture medium: use DMEM medium (manufactured by Invitrogen, product number 1280...
Embodiment 2
[0088] Influenza virus: H1N1 subtype influenza virus (see Chinese patent CN1644686A for this strain, which is deposited in China General Microorganism Collection and Management Center with the preservation number CGMCC No. 1014).
[0089] Serum-free cell culture medium: MEM medium (manufactured by GIBCO, product number 61100-087, batch number 678277) was prepared according to the product instructions, and the pH value was adjusted to 7.2.
[0090] Cell culture solution containing 8% (V / V) serum: use MEM medium (manufactured by GIBCO, product number 61100-087, batch number 678277) to prepare according to the product instructions, wherein 8% (V / V) newborn bovine serum ( Produced by GIBCO Company, product number 16010-14, batch number 693745), the pH value was adjusted to 7.2.
[0091] Serum-free cell culture medium containing TPCK trypsin: use MEM medium (manufactured by GIBCO, product number 61100-087, batch number 678277) to prepare according to the product instructions, add T...
Embodiment 3
[0098] Influenza virus: H5N3 subtype influenza virus (see Chinese patent CN1884498A for this strain, which is deposited in China General Microorganism Collection and Management Center with the preservation number CGMCC No. 1339).
[0099] Serum-free cell culture medium containing TPCK trypsin: use DMEM medium (manufactured by Invitrogen, product number 12800-82, batch number 309313) to prepare according to the product instructions, add TPCK trypsin (manufactured by Sigma-Aldrich company, product number T3053) to the end The concentration is 1mg / L, and the pH value is adjusted to 7.2.
[0100] Other reagents and materials used in this example are the same as Example 1.
[0101] The steps, methods and parameters of each culture stage used in this example are the same as those in Example 1.
[0102] Finally, 50L of the virus liquid was harvested. After testing, the HA was 1:1024, and the virus content was 10 8.5 TCID 50 / ml.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com