Self-assembling micelle-like nanoparticles for systemic gene delivery
A nanoparticle and carrier technology, applied in the field of self-assembled micelle-like nanoparticles for gene delivery in vivo, can solve the problems of complex and time-consuming preparation steps, low carrying capacity, long circulation time, etc., and achieve a repeatable preparation method, The effect of high load-carrying capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
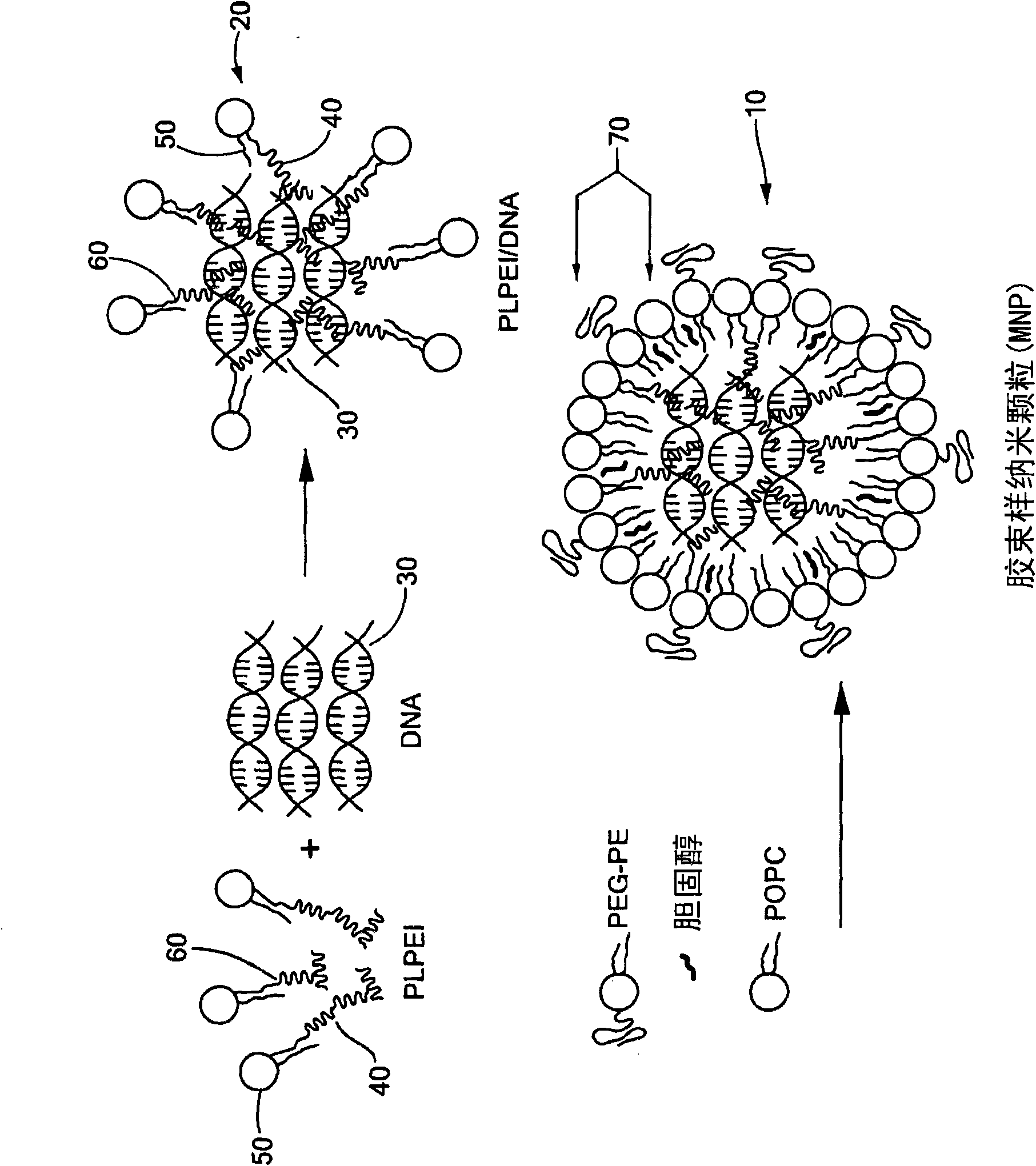
[0078] Preparation of micellar nanoparticles (MNPs)
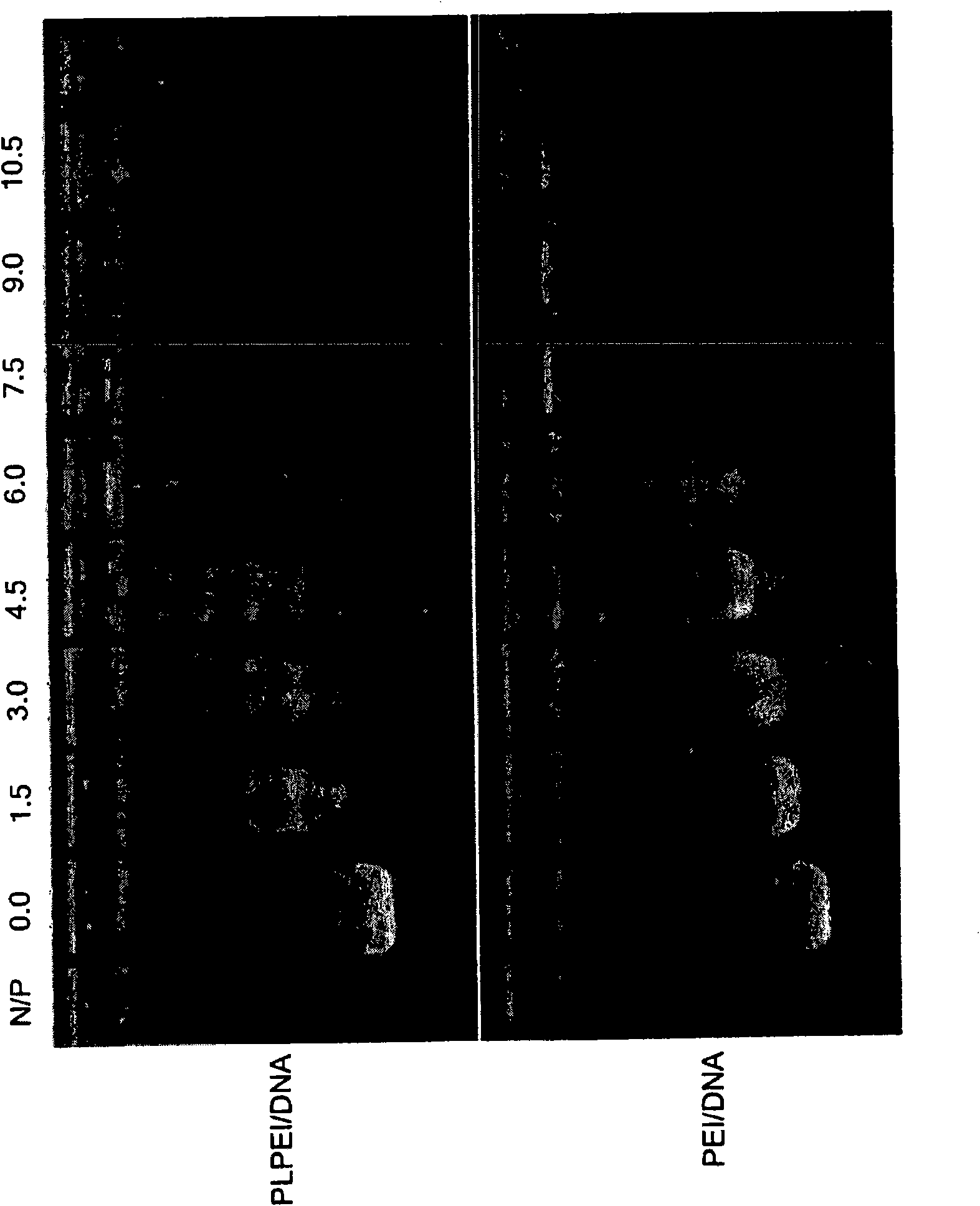
[0079] Preparation of micelle-like nanoparticles (MNP): Plasmid DNA and PLPEI form a complex, and then wrap the prepared complex with a lipid monolayer containing PEG-phosphatidylcholine conjugate (PEG-PE) ( figure 1 ). For complex formation, since complex formation will prevent DNA migration, retaining it in the wells, the optimal ratio of PLPEI to DNA is determined by the amount of amine required to completely inhibit DNA migration on an agarose gel . A certain amount of plasmid DNA was mixed with PLPEI at different amine / phosphorus ratios (N / P), and detected by agarose gel electrophoresis. The ratio of bound DNA increases with the increase of N / P ratio, and when the N / P ratio is higher than 6, most of the DNA is bound. Comparing the composite situation of PLPEI with that of non-modified PEI, it can be seen that conjugation with lipids does not reduce the ability of PEI to bind DNA ( Figure 2a ). An N / P ratio of 10 ...
Embodiment 2
[0083] Physicochemical characteristics of MNP
[0084] Traditional PEI / DNA complexes tend to aggregate rapidly under high-salt physiological conditions [8]. To demonstrate the stabilizing effect of the lipid outer membrane against salt-induced aggregation, NaCl was added to the complex formulation to a final concentration of 0.15M and the hydrodynamic diameter was monitored. As expected, PEI / DNA complexes rapidly aggregated upon addition of NaCl, with a sustained increase in hydrodynamic diameter up to nearly 20-fold within 24 h. The diameter of the PLPEI / DNA complex intermediate without free lipid and PEG-PE rapidly increased by 2-fold upon the addition of NaCl, and then remained relatively constant within 24 hours. In contrast, MNPs remained stable after the addition of salt, with no apparent aggregation within 24 h ( Figure 3a ).
[0085] Zeta potential measurements revealed that MNPs have a favorable neutral surface charge ( ) of −2.1±0.86 mV (mean±s.e.m., n=5), while ...
Embodiment 3
[0089] In vivo biodistribution and gene expression
[0090] In order to show the prolonged circulation time of MNP in the blood and the resulting higher feasibility of delivering it to target organs such as tumors, mice carrying 111 In-DNA MNP was studied for pharmacokinetics and biodistribution. IV bolus carry 111 After In-DNA MNP, radioactivity in major organs was measured and compared with control PEI / 111 In-DNA for comparison. After 10 minutes, as much as 30% ID / ml of MNP was still in the blood, compared to about 10% ID / ml for PEI / DNA. One hour after injection, about 20% ID / ml of MNP was still in the blood, while only about 5% ID / ml of PEI / DNA could be detected in the circulation (Fig. 5A).
[0091] The slower clearance of MNP-encapsulated DNA relative to PEI / DNA and the resulting prolongation of circulation time were also confirmed by pharmacokinetic parameters. The half-life (t 1 / 2β ), the half-life was found to be approximately 239 minutes, compared to 33 minutes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com