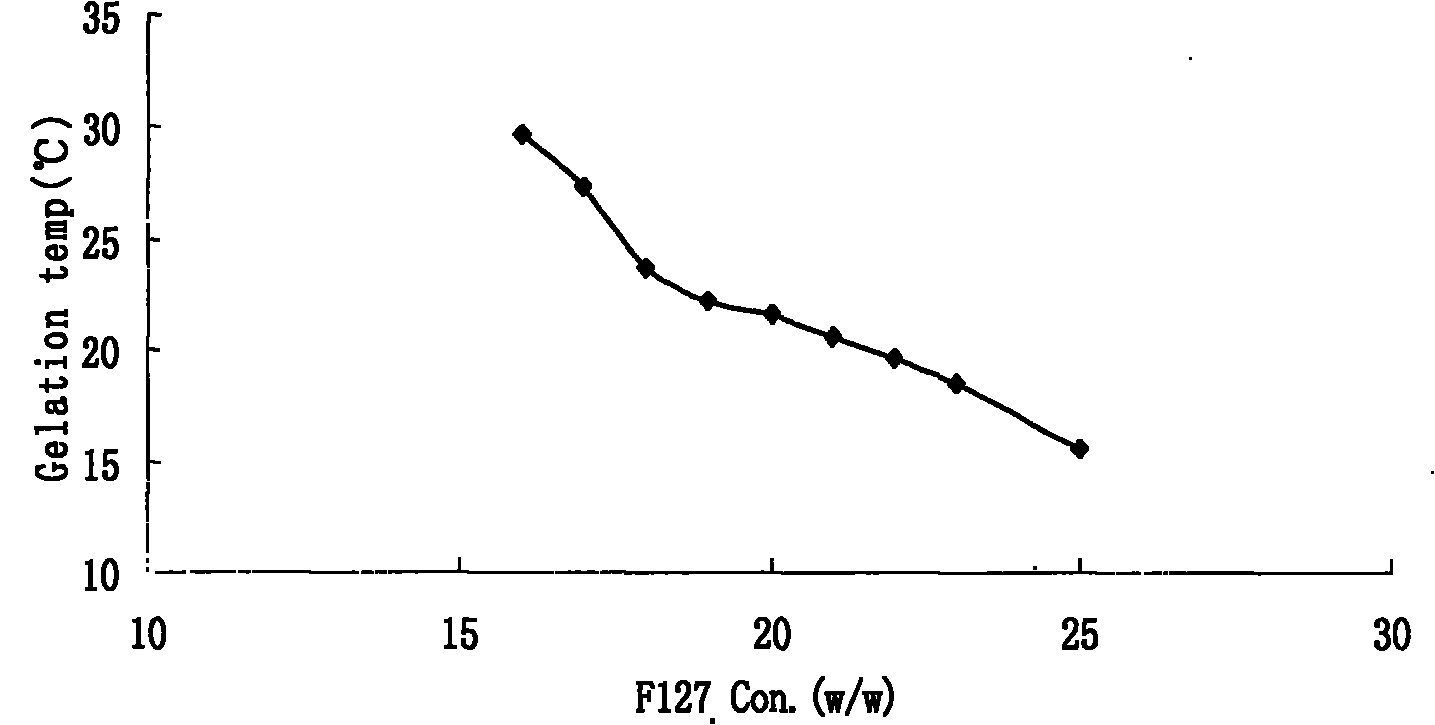
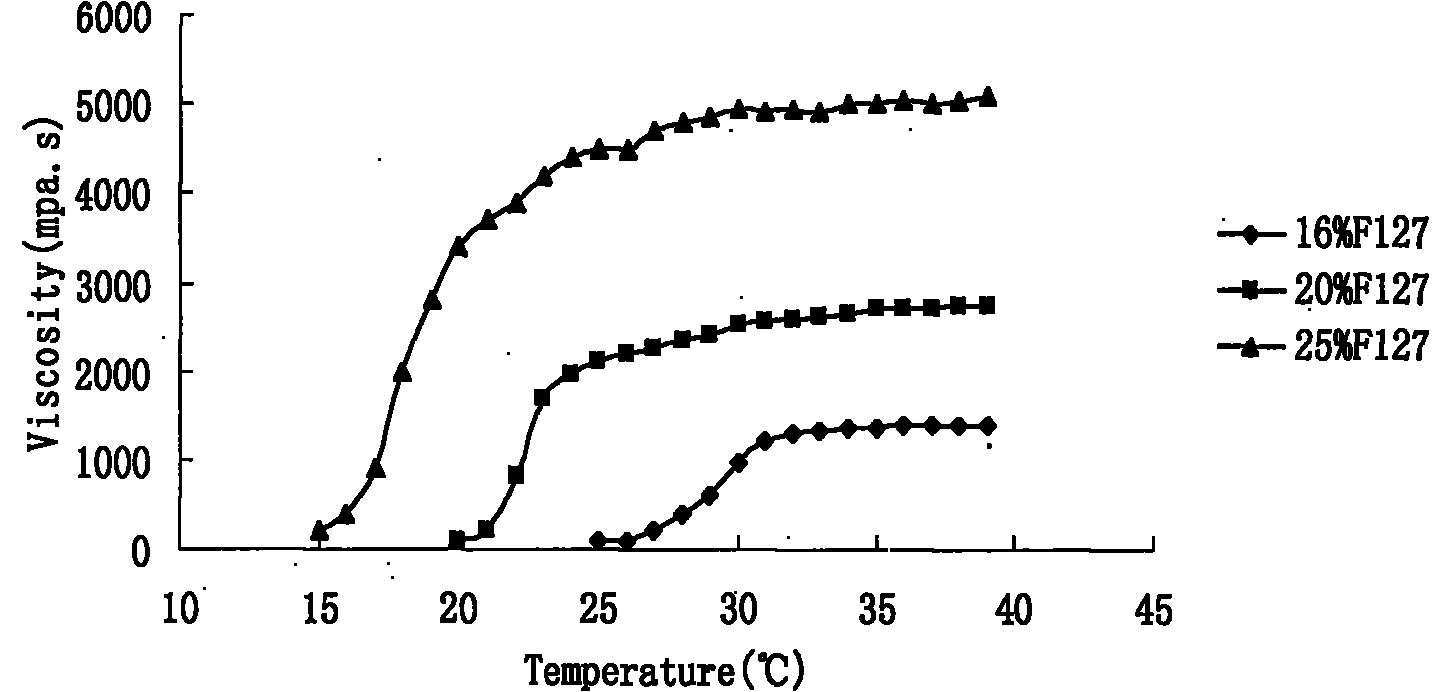
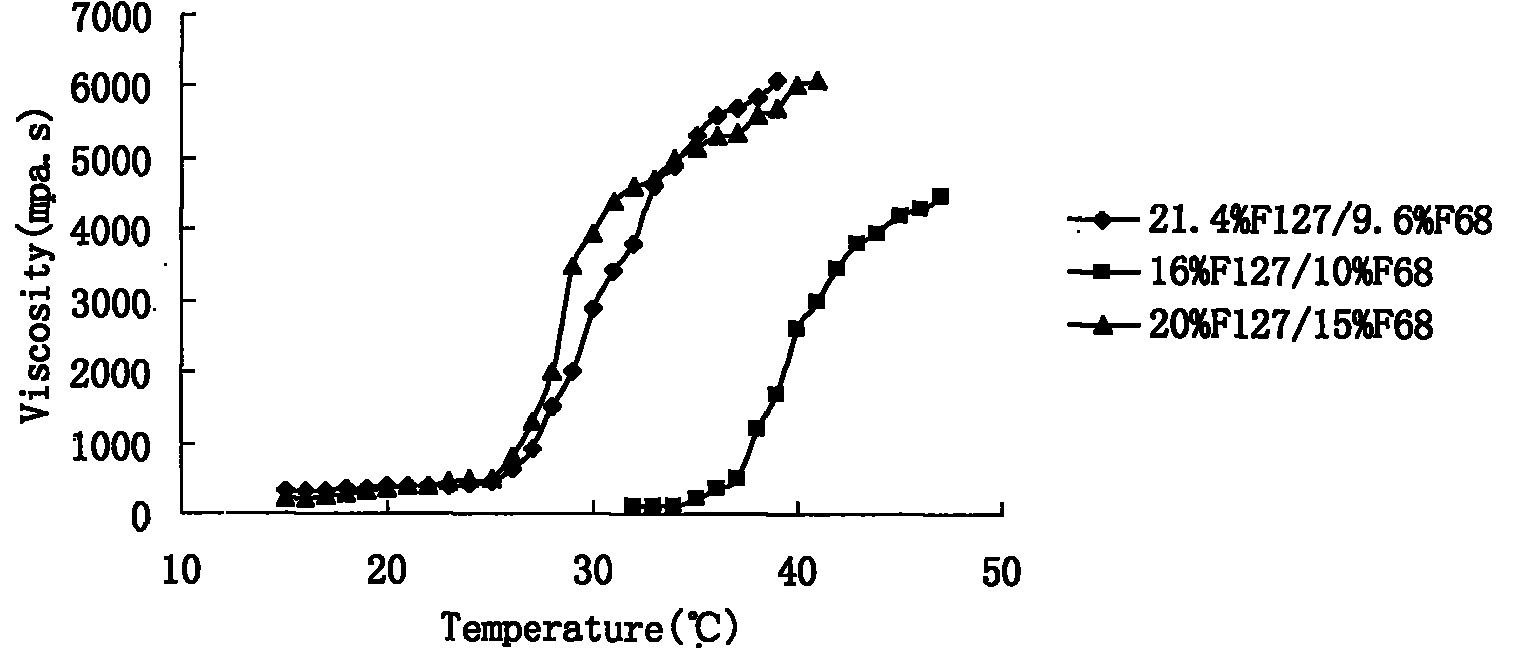
Preparation and application of olopatadine in-situ gel
A technology of in-situ gel and olopatadine, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, respiratory diseases, etc., can solve the problem of high acid carbomer concentration, discomfort, and eye problems Irritation and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Embodiment 1 Preparation of olopatadine thermosensitive eye (or nasal cavity) gel
[0098] [Prescription] Olopatadine 0.15g
[0099] Brogeramine (Benzalkonium Bromide) 0.02g
[0100] F127 21.4g
[0101] F68 9.6g
[0102] Add isotonic phosphate buffer (pH7.4) to the full amount of 100g
[0103] [Preparation method] Take olopatadine and dissolve it in 60% sterile isotonic phosphate buffer (pH7.4) with prescribed amount of bromogeramine, slowly add the prescribed amount of F127 and F68 under stirring condition, add isotonic phosphate buffered saline To a sufficient amount, store in cold storage at low temperature to fully swell until a clear and uniform solution is formed.
Embodiment 2
[0104] Embodiment 2 Preparation of olopatadine thermosensitive ophthalmic (or nasal cavity) gel
[0105] [Prescription] Olopatadine 0.1g
[0106] Brogeramine (Benzalkonium Bromide) 0.02g
[0107] F127 21.4g
[0108] F68 9.6g
[0109] NaCl 0.9g
[0110] Add distilled water to the full amount of 100g
[0111] [Preparation method] Dissolve olopatadine, brogeramine, and NaCl in 60% distilled water of the prescribed amount, slowly add the prescribed amount of F127 and F68 under stirring conditions, add distilled water to a sufficient amount, and store in low temperature refrigeration to fully swell until it becomes clear homogeneous solution.
Embodiment 3
[0112] Embodiment 3 Preparation of olopatadine thermosensitive ophthalmic (or nasal cavity) gel
[0113] [Prescription] Olopatadine 0.3g
[0114] Brogeramine (Benzalkonium Bromide) 0.02g
[0115] F127 21.4g
[0116] F68 9.6g
[0117] Mannitol 5g
[0118] Add distilled water to the full amount of 100g
[0119] [Preparation method] Dissolve olopatadine, brogeramine, and mannitol in 60% distilled water of the prescribed amount, slowly add the prescribed amount of F127 and F68 under stirring conditions, add distilled water to a sufficient amount, and store in low temperature refrigeration to fully swell until formed Clear homogeneous solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com