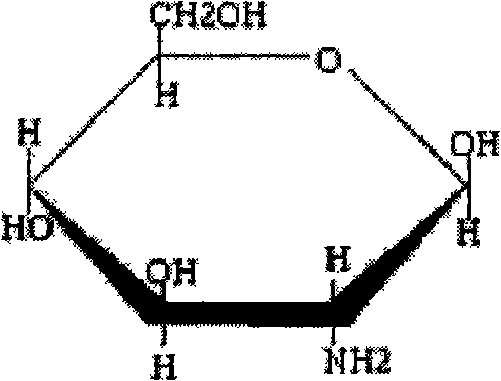
Glucosamine formulations
A technology of glucosamine and glucose, applied in anti-inflammatory agents, pill delivery, non-central analgesics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 - Glucosamine Oral Solution Containing Chitosan as Absorption Promoter
[0060] Glucosamine oral solutions containing chitosan as an absorption enhancer were prepared according to the following Table 1, and packaged in conventional oral liquid bottles or plastic bags.
[0061]Table 1 - Examples of oral solution formulations containing glucosamine and the absorption enhancer chitosan
[0062]
[0063]
[0064] Preparation 2
[0065]
[0066] Preparation 3
[0067]
[0068] Preparation 4
[0069]
Embodiment 2
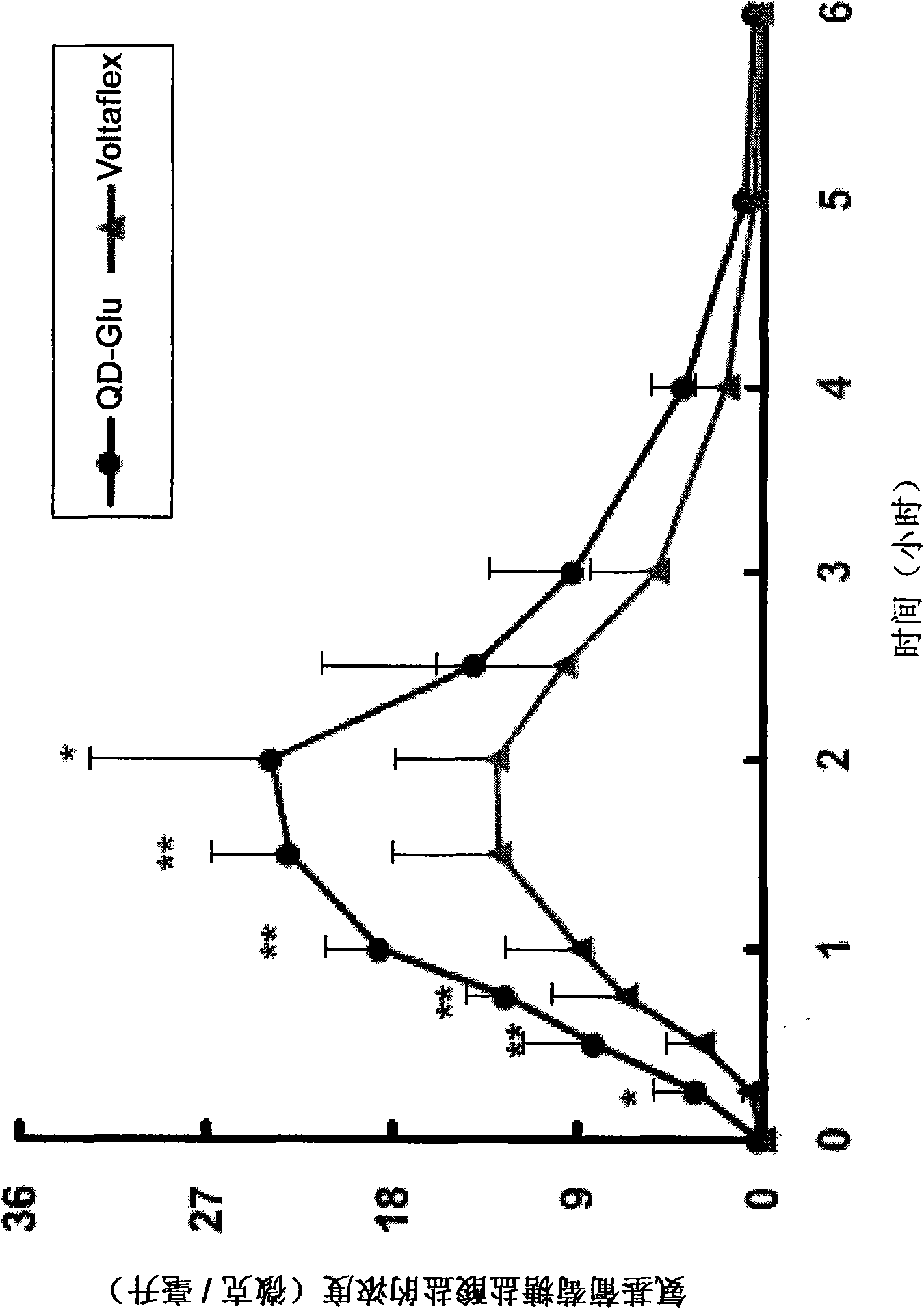
[0070] Example 2 - Formulations containing chitosan as a glucosamine absorption enhancer against rats and hunting animals Pharmacokinetic properties of rabbits and dogs
[0071] With rats and Beagle dogs, the bioavailability of the preparation 2 (QD-Glu solution) of embodiment 1 and the commercially available solution (Wellesse TM The bioavailability of glucosamine hydrochloride 2000mg / 30ml) was compared.
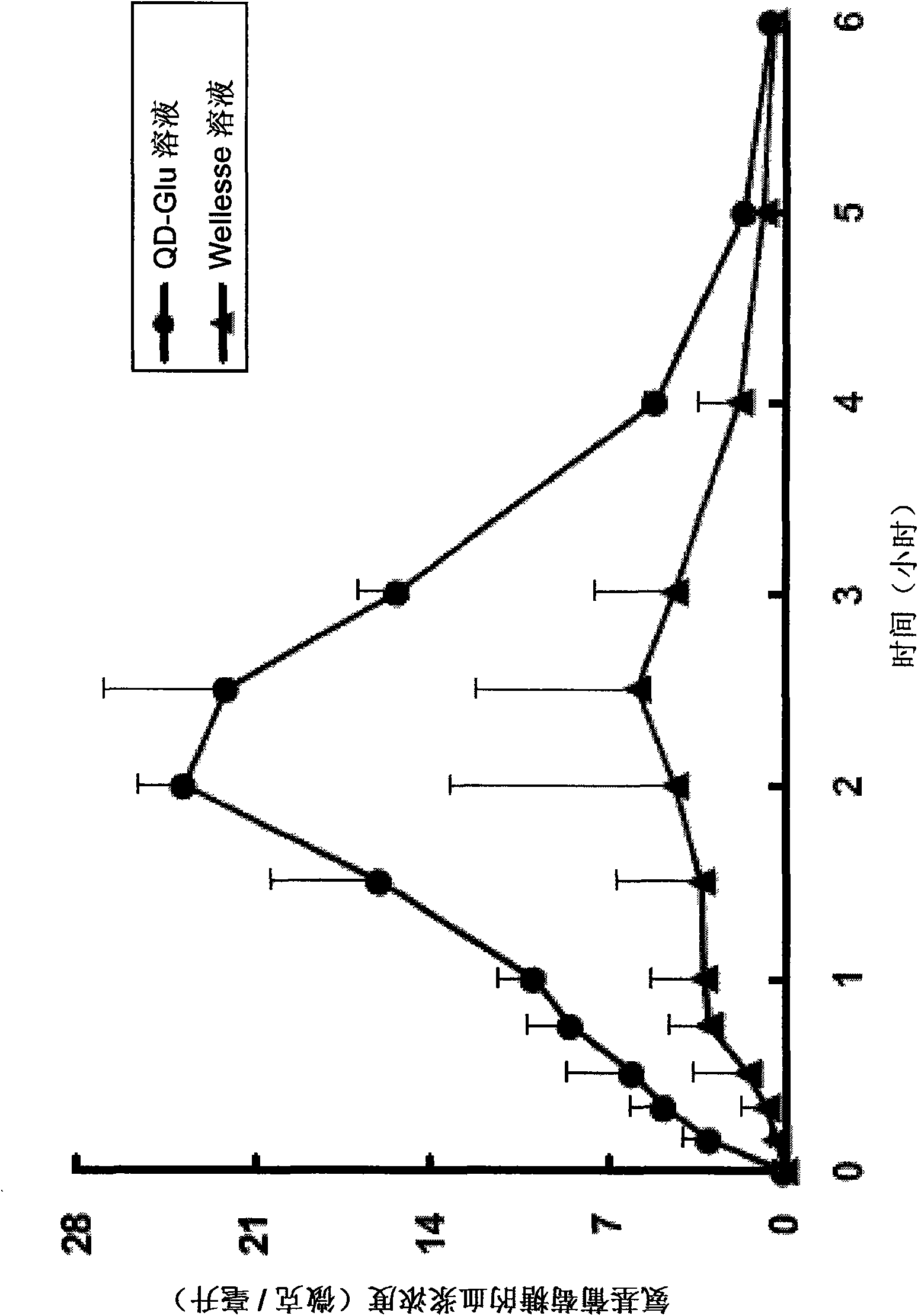
[0072] Rat test: According to the dose of 360 mg / kg body weight, eight Sprague-Dawley male rats with a body weight of 225 to 250 g were orally administered the test preparation (QD-Glu solution) or the reference preparation by gavage (Wellesse TM solution). Blood samples were drawn at selected time points after each treatment. Determination of blood concentration levels of glucosamine of test drug and reference drug, and shown in figure 1 middle.
[0073] with Wellesse TM Rats administered the glucosamine formulation of the present invention showed higher plasma d...
Embodiment 3
[0080] Example 3 - Glucosamine oral solution containing cyclodextrin as absorption enhancer
[0081] Glucosamine oral solutions containing cyclodextrin as an absorption enhancer were prepared according to the following Table 3, and packaged in conventional oral liquid bottles or plastic bags.
[0082] Table 3 - Examples of oral solution formulations containing glucosamine and the absorption enhancer cyclodextrin
[0083]
[0084] Hydroxypropyl-β-CD, dimethyl-β-CD, and sulfobutyl ether-β-CD were accurately weighed and dissolved in water, respectively. Glucosamine hydrochloride, preservatives, sweeteners and flavorings are added and stirred for a certain period of time until a clear solution is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com