Bupropion hydrochloride sustained release tablets and preparation method thereof
A technology of bupropion hydrochloride and sustained-release tablets, which is applied to medical preparations with non-active ingredients, pharmaceutical formulas, and medical preparations containing active ingredients, etc. It can solve the problem of large differences between product batches and easy-to-corrosion production equipment , Unfavorable for industrialized production and other issues, to achieve the effect of easy identification, no pollution to the environment, and conducive to industrialized production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
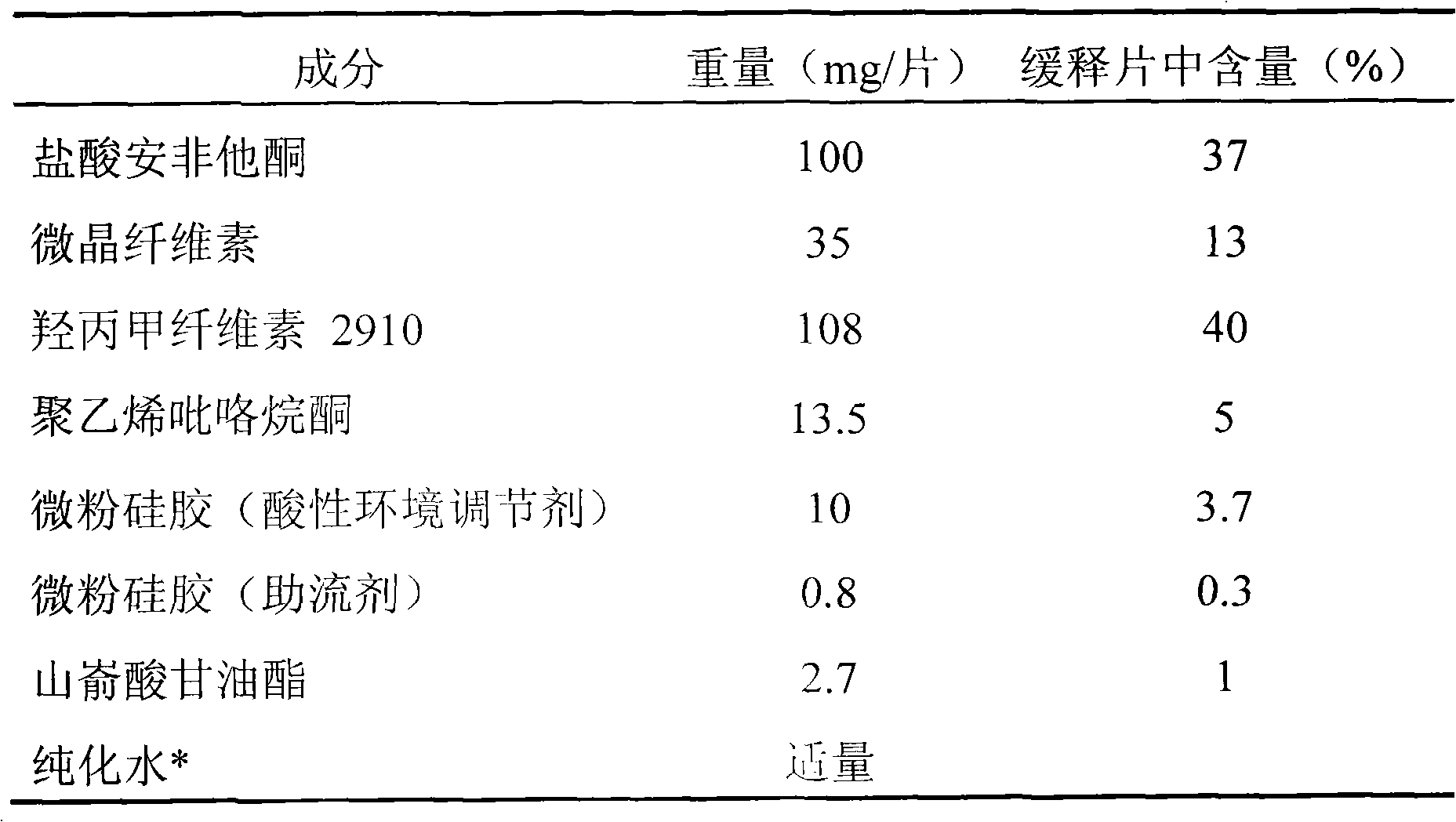
[0027] Embodiment 1 prepares the sustained-release tablet containing 100mg bupropion hydrochloride
[0028] Table 1
[0029]
[0030] *Removable after drying
[0031] Take by weighing bupropion hydrochloride, hypromellose, microcrystalline cellulose, polyvinylpyrrolidone, acidic environment modifier micropowder silica gel (10mg / sheet, accounting for 3.7% of the sustained-release tablet weight) according to Table 1, after mixing , Use an appropriate amount of water to make granules. After the wet granules are dried and sized, add glyceryl behenate and glidant micropowder silica gel (0.8 mg / tablet, accounting for 0.3% of the weight of the sustained-release tablet), mix well, and press into tablets to obtain the product. Coat the plain tablets with a film, increase the weight to 2%-3%, and pack them separately.
Embodiment 2
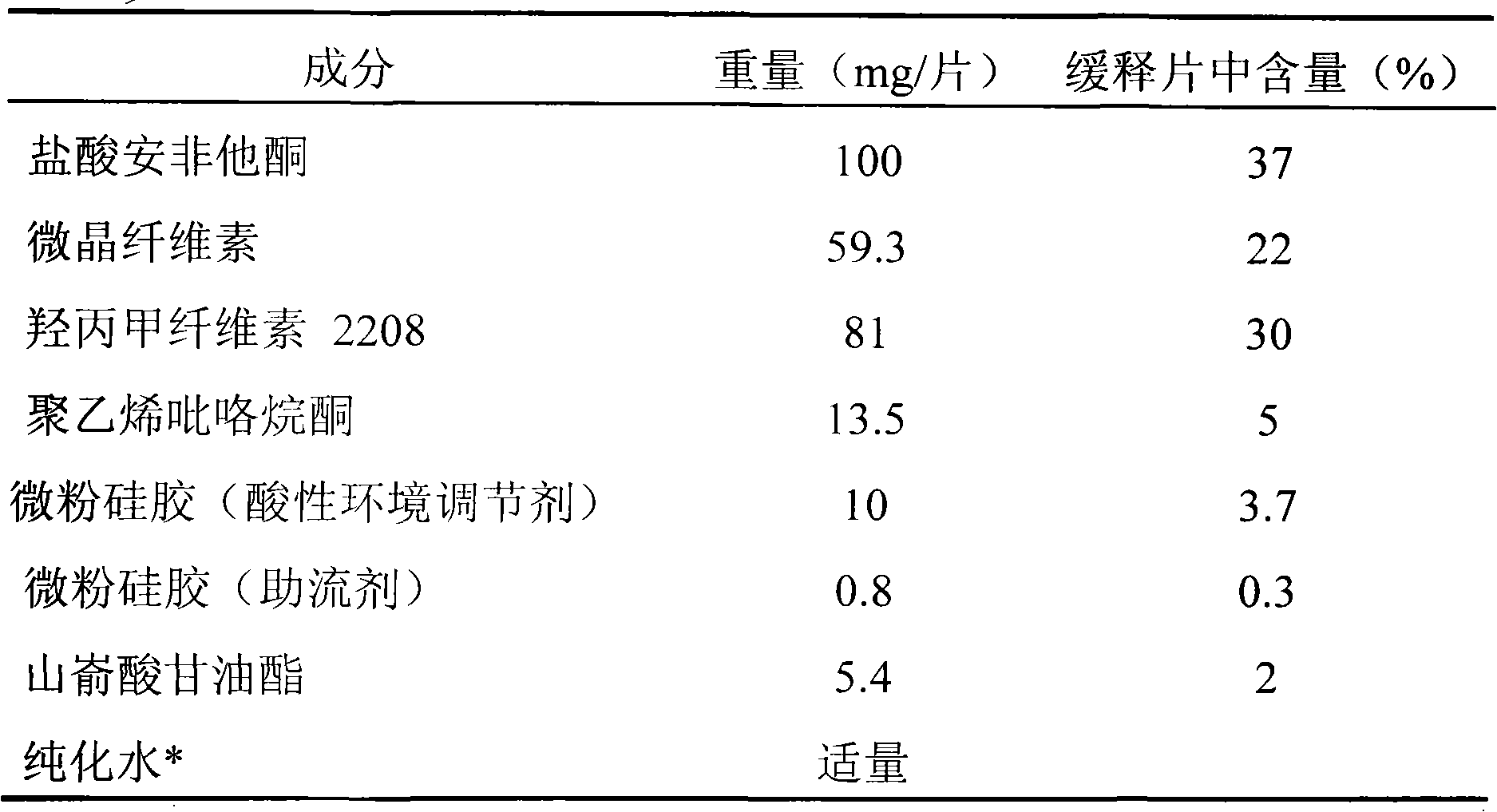
[0032] Example 2 Preparation of sustained-release tablets containing 100mg bupropion hydrochloride
[0033] Table 2
[0034]
[0035] *Removable after drying
[0036] Take by weighing bupropion hydrochloride, hypromellose, microcrystalline cellulose, polyvinylpyrrolidone, acidic environment modifier micropowder silica gel (10mg / sheet, accounting for 3.7% of the sustained-release tablet weight) according to Table 2, after mixing , Use an appropriate amount of water to make granules. After the wet granules are dried and sized, add glyceryl behenate and glidant micropowder silica gel (0.8 mg / tablet, accounting for 0.3% of the weight of the sustained-release tablet), mix well, and press into tablets to obtain the product. Coat the plain tablets with a film, increase the weight to 2%-3%, and pack them separately.
Embodiment 3
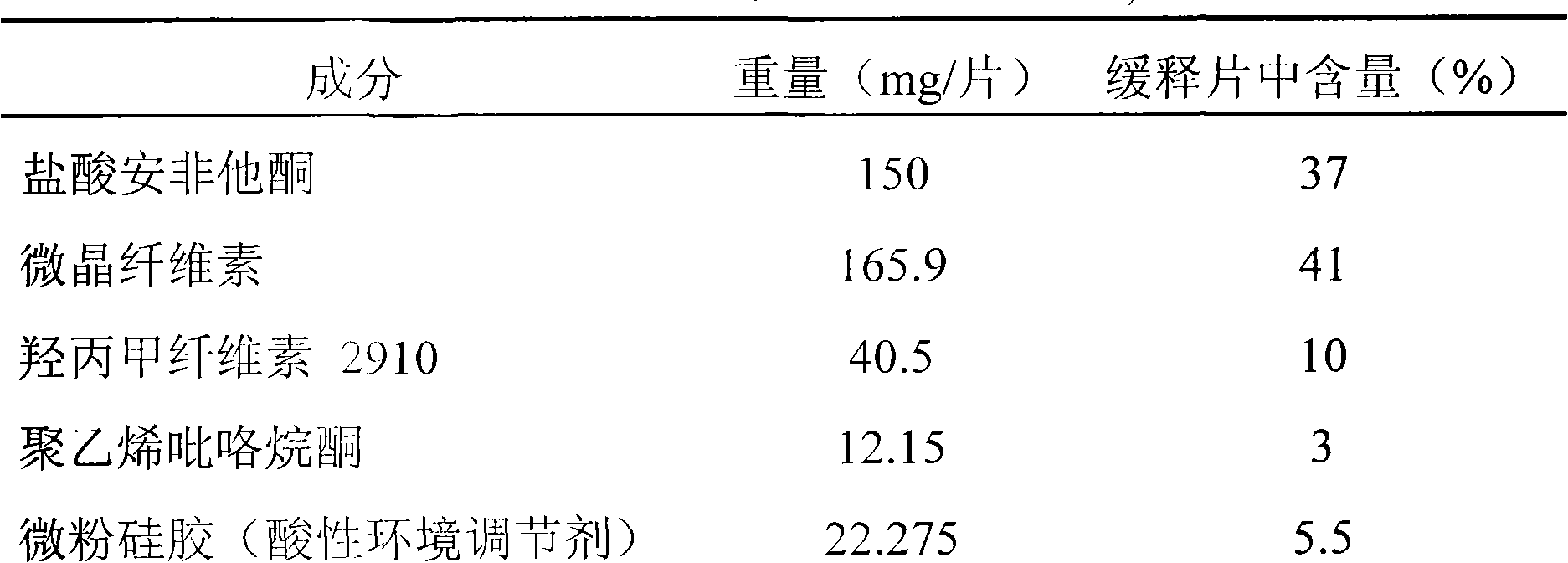
[0037] Example 3 Preparation of sustained-release tablets containing 150mg bupropion hydrochloride
[0038] table 3
[0039]
[0040]
[0041] *Removable after drying
[0042] Take by weighing bupropion hydrochloride, hypromellose, microcrystalline cellulose, polyvinylpyrrolidone, acidic environment modifier micropowder silica gel (22.275mg / sheet, accounting for 5.5% of the sustained-release tablet weight) according to Table 3, and mix well Finally, make granules with appropriate amount of water. After the wet granules are dried and sized, add glyceryl behenate and glidant micropowder silica gel (2.025 mg / tablet, accounting for 0.5% of the weight of the sustained-release tablet), mix well, and press into tablets. Coat the plain tablets with a film, increase the weight to 2%-3%, and pack them separately.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com