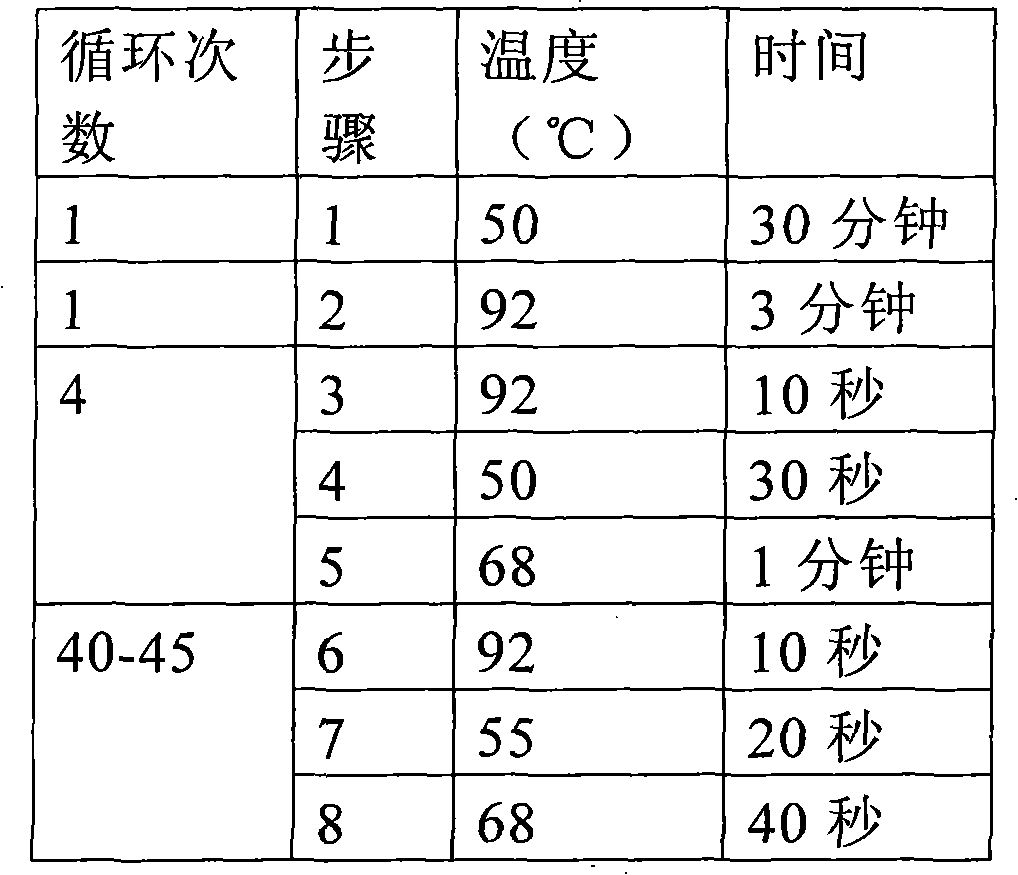
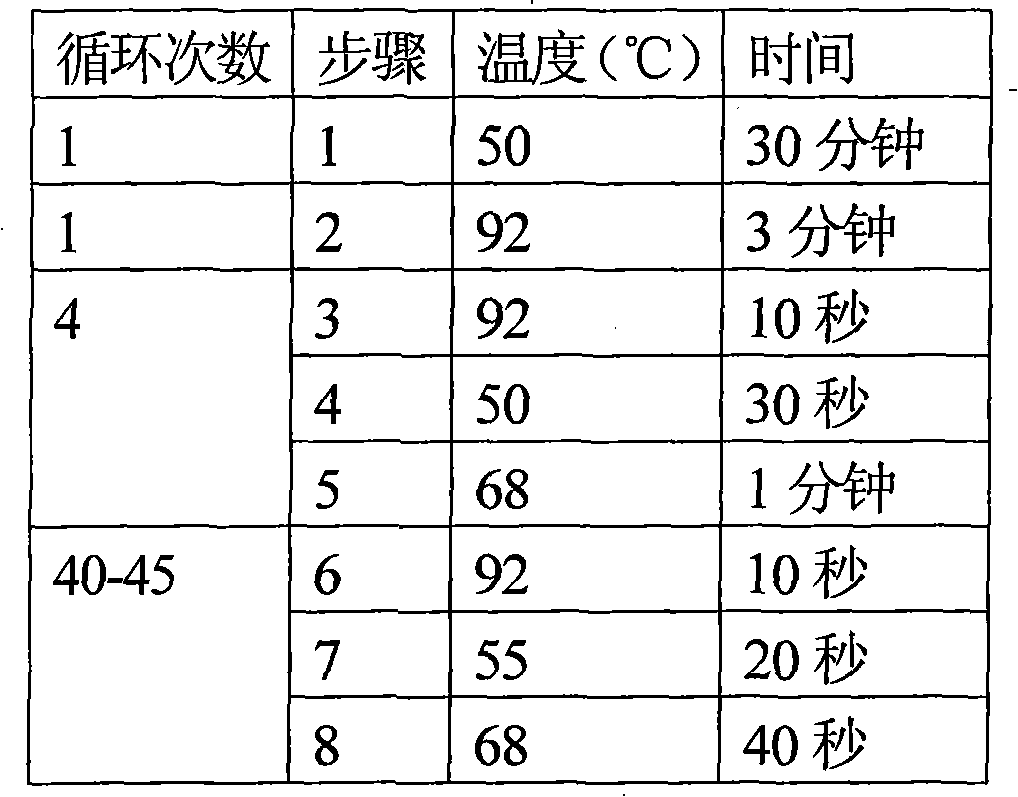
Fluorescent quantitative PCR (Polymerase Chain Reaction) detection kit and detection method of various viruses of donor corneas
A detection kit and donor technology, which is applied in the field of molecular biology detection of corneal virus infection in donors, can solve the problems of PCR false positive pollution, ordinary PCR false positive pollution, and difficulty in improving quantitative accuracy, and achieve quantitative accuracy High, to avoid the effect of cross-contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 Donor corneal HCV virus detection:
[0060] 1. Extraction of total RNA from the conjunctival tissue of the donor eye and construction of a positive working standard:
[0061] (1) Take 5 mg of donor eyeball conjunctival tissue, put the tissue block into a centrifuge tube, add 350 μL Trizol, and fully homogenize with an electric homogenizer for 1-2 minutes. Place at room temperature for 5 minutes to fully lyse;
[0062] (2) Put the tissue lysate into the filter column and centrifuge at 12000rpm for 1min. Discard the filter column, add 350 μL of 70% ethanol to the filtrate, and repeatedly pipette 5 times to mix;
[0063] (3) Add the sample to the adsorption column and centrifuge at 12000rpm for 30s. Replace with a new filtrate collection tube, add 350 μL filter membrane desalting solution, and centrifuge at 12000 rpm for 1 min;
[0064] (4) Add 95 μL DNase reaction solution and let stand at room temperature for 5 minutes. Add 200 μL of eluent 2 and centrifug...
Embodiment 2
[0081] Example 2 Donor corneal HIV1 virus detection:
[0082] 1. Extraction of total RNA from the conjunctival tissue of the donor eye and construction of a positive working standard: the specific steps are the same as the extraction of total RNA from the conjunctival tissue of the donor eye in Example 1.
[0083] A plasmid (pUC57-HIV1) containing the HIV1 gene was constructed as a positive working standard. The positive working standard contains the target sequence amplified by the forward and reverse primers of HIV1. In addition, a sequence is added on both sides of the target sequence to make it closer to the structure of the actual test sample, so as to ensure the consistency with the actual test sample. Consistency of amplification efficiency.
[0084] Using the Promega PureYield plasmid miniprep system kit, extract the HIV1 positive working standard plasmid, and use a UV spectrophotometer to compare the color at 260nm and 280nm to obtain the purity and concentration of ...
Embodiment 3
[0097] Example 3 Donor corneal RV virus detection:
[0098] 1. Extraction of total RNA from the conjunctival tissue of the donor eye and construction of a positive working standard: the specific steps are the same as the extraction of total RNA from the conjunctival tissue of the donor eye in Example 1.
[0099] A plasmid (pUC57-RV) containing the RV gene was constructed as a positive working standard. Each positive working standard contains the target sequence amplified by RV forward and reverse primers. In addition, a sequence is added on both sides of the target sequence to make it closer to the structure of the actual detection sample to ensure that it is consistent with the actual detection sample. The consistency of the amplification efficiency among them.
[0100] Use the Promega PureYield plasmid miniprep system kit to extract the RV positive working standard plasmid, and use a UV spectrophotometer to compare colors at 260nm and 280nm to obtain the purity and concentr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com