Reduction sensitive polyethyleneimine derivative as well as preparation method and application thereof
A polyethylenimine and sensitive technology, which is applied in the field of reduction-sensitive polyethyleneimine derivatives and their preparation and application, can solve the problem of reducing the ability of polymer binding and protecting DNA, low yield, complicated process, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
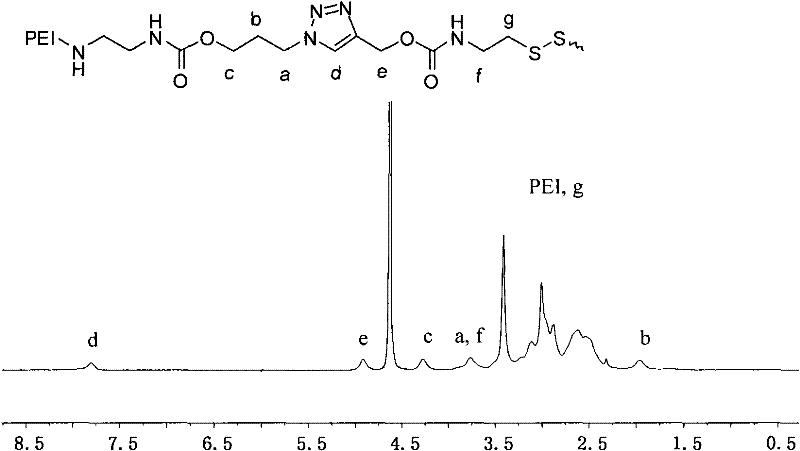
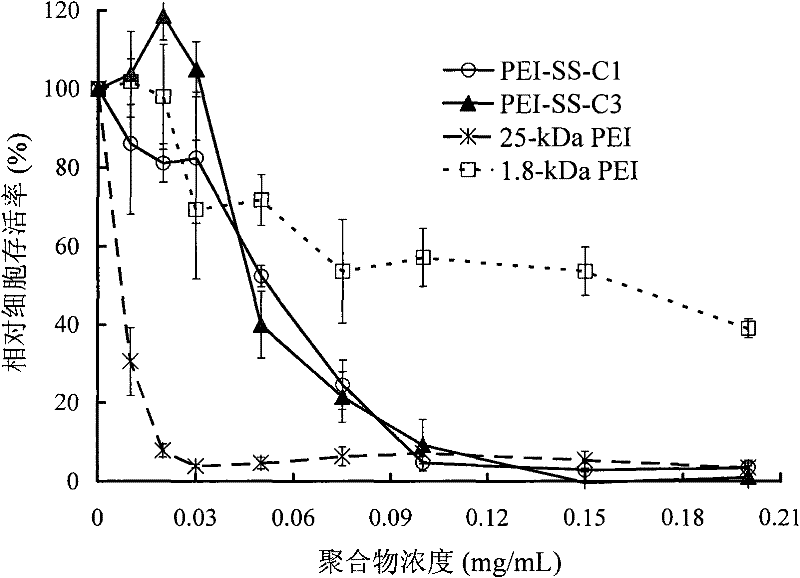
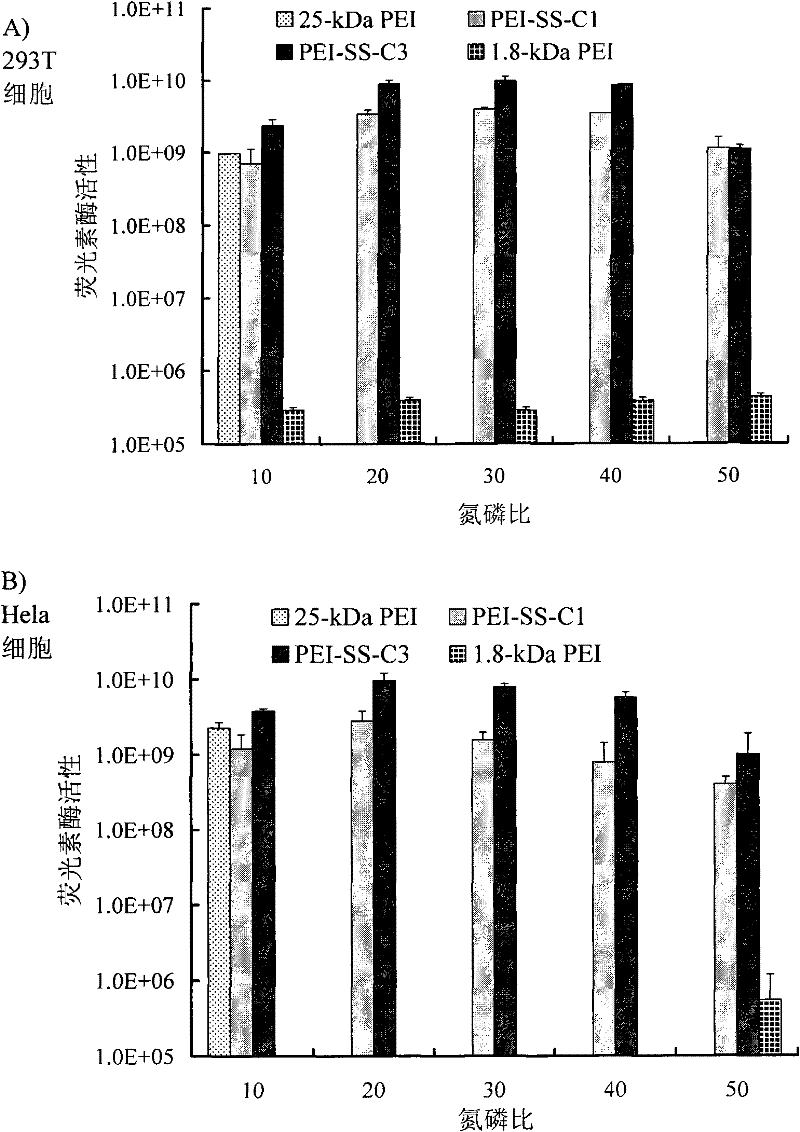
[0065] The above-mentioned reduction-sensitive micro-crosslinked modified polyethyleneimine derivatives of the present invention (using the simplified formula PEI-(ASSA-PEI) n indicated that PEI 1800 -(ASSA-PEI 1800 ) n , representing that the weight-average molecular weight that it uses PEI is 1800, also expresses with PEI-SS-C) preparation method, comprises following three steps:
[0066]
[0067]In step a) above, a disulfide bond-containing chain extender (crosslinking agent) can be prepared: compound 1 propargyl carbonylimidazole and cystamine dehydrochloride are added in a molar ratio of 1-4:1 Proton-free organic solvents such as chloroform, dichloromethane, acetone, tetrahydrofuran, DMF, stirred at 20-60 ° C for 2-48 hours, purified to obtain bis (allyl carbamate ethyl) disulfide ( bis[(propargyl carbamate) ethyl] disulfide, BPPA-Cyst, compound 2) (see Example 1), can also adjust the feed molar ratio of compound 1 and cystamine to be 0.5-1.5: 1 to prepare (allylami...
Embodiment 1
[0088] Example 1 Synthesis of two (allyl carbamate ethyl) disulfide (bis[(propargyl carbamate) ethyl] disulfide, BPPA-Cyst, 2):
[0089] According to the literature [X.L.Jiang, M.C.Lok, W.E.Hennink, Degradable-BrushedpHEMA-pDMAEMA synthesized via ATRP and click chemistry for gene delivery, Bioconjugate Chem.18(2007) 2077-2084] through propargyl alcohol and 1,1'-carbonyldiimidazole Synthesis of propargyl ester of carbonyl-imidazole (PPA-CI, 1). First desalt cystamine hydrochloride, weigh 4.40 g of dehydrochlorated cystamine and 6.93 g of propargyl carbonylimidazole in 50 ml of dichloromethane, stir and react at room temperature for 24 hours, remove the solvent, add 100 ml of phosphoric acid Sodium dihydrogen solution (pH 4.0) was dissolved, and then extracted 3 times with ether to obtain BPPA-Cyst with a yield of 80%. 1 H-NMR in CDCl 3 : δ(ppm) 2.48(s, 2H, CH≡C), 2.82(t, 4H, S-CH 2 -C), 3.51 (app.quartet, 4H, NH-CH 2 -C), 4.69(s, 4H, O-CH 2 -C≡C), 5.31 (s, 2H, NH-C=O). Inf...
Embodiment 2
[0090] Embodiment 2 (allylcarbamate ethyl) dithioethyl 1-carboxamide imidazole ((propargylcarbamate) ethyl disulfide ethyl 1-carbamide-imidazole, PPA-Cyst-CI, 7) is first synthesized (ene Propyl carbamate ethyl) dithioethylamine ((propargyl carbamate) ethyl disulfideethylamine, PPA-Cyst, 6): Propargyl carbamate carbonylimidazole (PPA-CI, 1) was synthesized according to the steps in Example 1. First desalt cystamine hydrochloride, weigh 4.40 grams of cystamine and 3.47 grams of propargyl carbonylimidazole in 50 milliliters of chloroform, stir and react at room temperature for 24 hours, remove the solvent, add 80 milliliters of sodium dihydrogen phosphate solution (pH 4.0 ) was dissolved, extracted 3 times with diethyl ether, adjusted the pH of the obtained lower aqueous phase to 9.0, then extracted 3 times with 40 ml of ethyl acetate, combined the organic phases, washed once with 40 ml of pH=9 sodium phosphate solution, and obtained The organic phase was dried with anhydrous ma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com