Mercury-free Japanese encephalitis inactivated vaccine composition and application thereof
A technology of inactivated vaccines and compositions, applied in the field of vaccine compositions and Japanese encephalitis vaccine compositions, to achieve the effect of ensuring good health
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1 mercury-free vaccine
[0035] Take a certain volume of the vaccine stock solution, dilute it 5, 10, 15, and 20 times with the phenol red-free 199 culture medium with a diluent of 2.5% (v / v) 20% human serum albumin, and neutralize it with immunized mice. The titer is determined by antibody assay (the conformity assessment standard is: T≥(RA+RB) / 2—0.33, wherein: T=tested vaccine, RA / RB=reference vaccine). Determine that a certain dilution factor falls within the range of the target titer determination (take the highest dilution factor among all qualified data). The phenol red-free 199 culture solution and the virus protection agent (human serum albumin) were added to the vaccine stock solution according to the dilution factor to obtain the mercury-free JE inactivated vaccine.
[0036] Take the preparation of 10 liters of vaccine liquid as an example, if the dilution factor measured by the qualified experiment is 10 times, in 1 liter of vac...
Embodiment 2
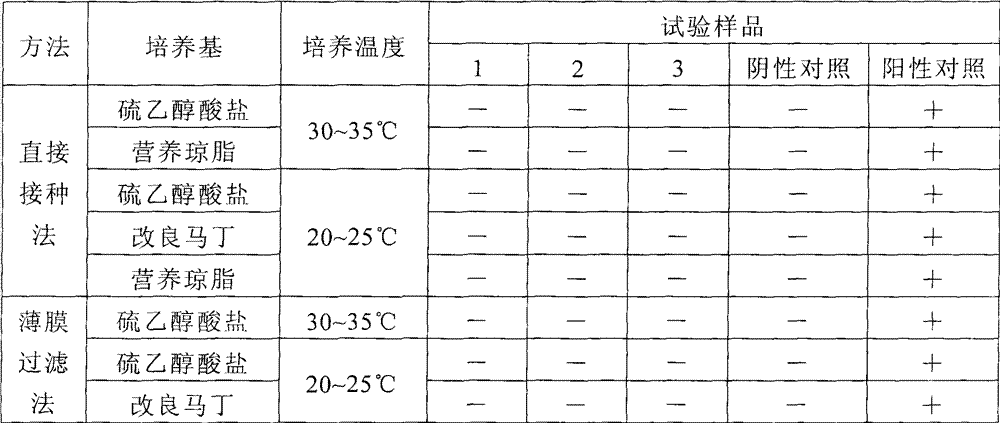
[0042] Example 2 Bacterial inspection of mercury-free vaccines and mercury-containing vaccines
[0043] Prepare the vaccine solution according to the prescription of this preparation, and compare it with the vaccine solution supplemented with 0.01% (v / v) thimerosal and the inactivated Japanese encephalitis vaccine (produced by Beijing Tiantan Biological Company, containing 0.01% thimerosal) in the existing market respectively. Parallel sterility test verification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com