Organic electroluminescent compounds and organic light emitting diode using the same
A compound, electroluminescence technology, applied in the direction of organic chemistry, compounds of elements of Group 4/14 of the periodic table, luminescent materials, etc., can solve problems such as hindering power consumption, small improvement of operating voltage, deterioration and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
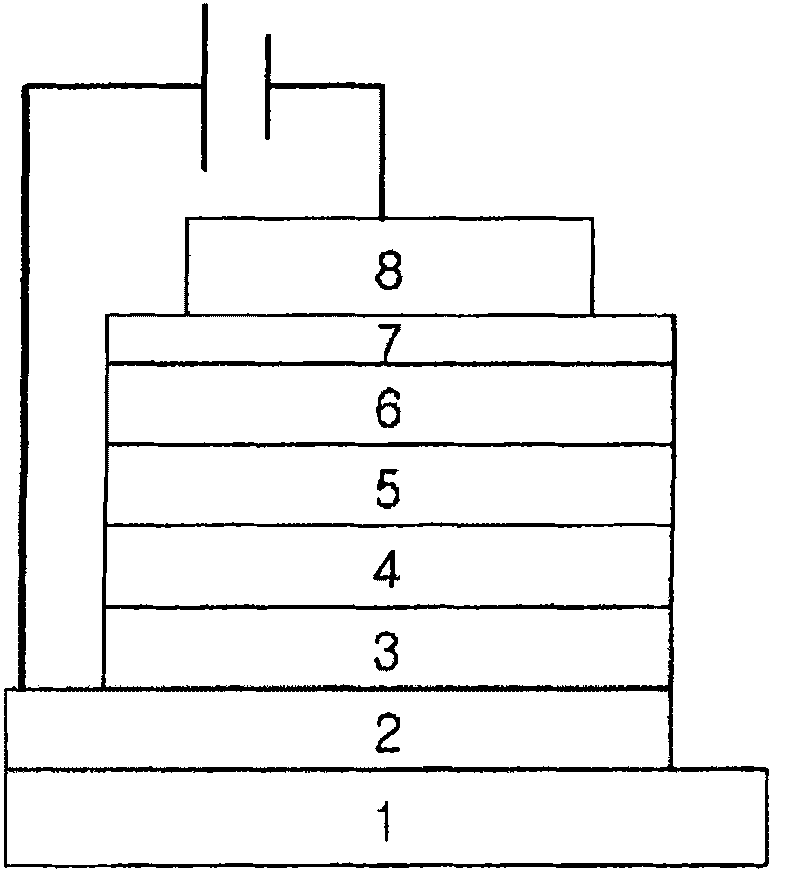
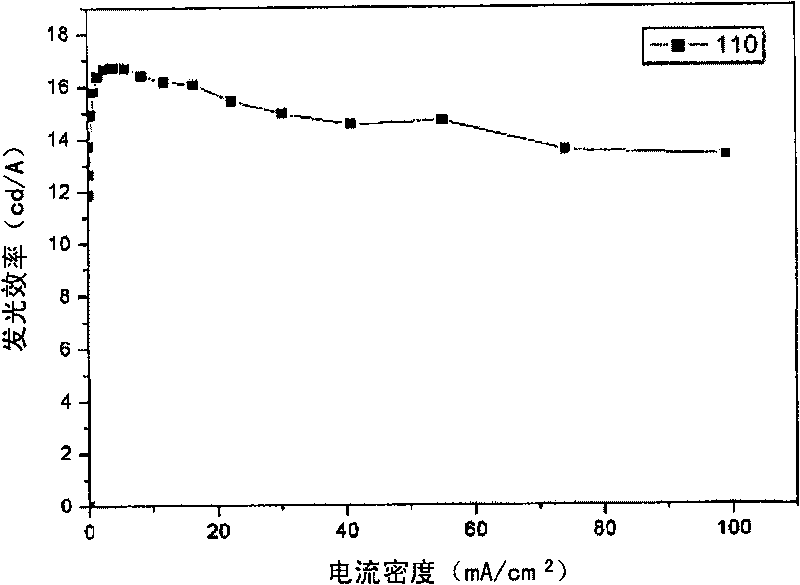
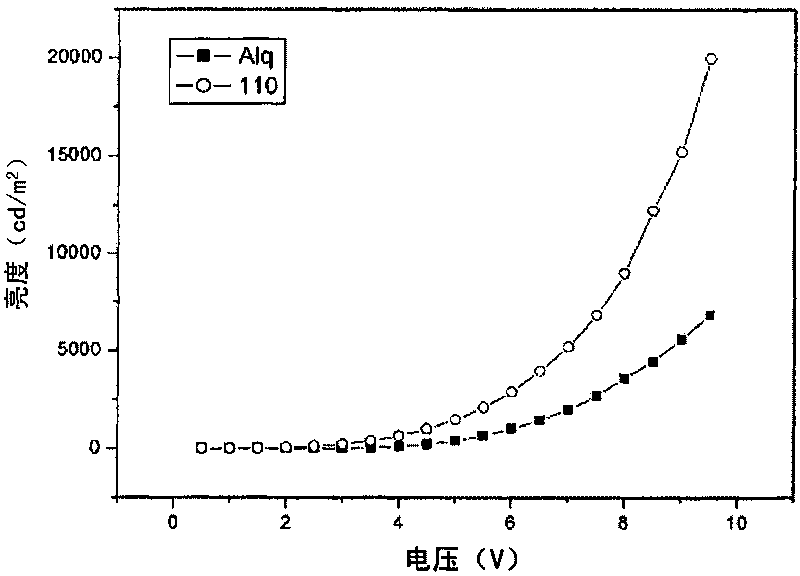
Image
Examples
preparation example 1
[0110] [Preparation Example 1] Preparation of Compound (102)
[0111]
[0112] Preparation of compound (201)
[0113] Add 1,2-dibromobenzene (100.0 g, 423.9 mmol), 2-naphthaleneboronic acid (80.2 g, 466.3 mmol), toluene (1000 ml) and tetrakis(triphenylphosphine) palladium (Pd( PPh 3 ) 4 ) (24.5 g, 21.2 mmol), and the mixture was stirred under an argon atmosphere. Aqueous potassium carbonate solution (300 ml) was then added dropwise to the mixture, and the resulting mixture was heated under reflux with stirring for 4 hours. The reaction was quenched by adding distilled water (2000 mL), and the reaction mixture was extracted with ethyl acetate (1000 mL). The organic extract was dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. Purification by silica gel column chromatography (ethyl acetate:hexane=1:50) gave 1-bromo-2-(2-naphthyl)benzene (63.59 g, 224.7 mmol, yield: 53.0%).
[0114] 1-Bromo-2-(2-naphthyl)benzene (42.0 g, 148.5 mm...
preparation example 2
[0125] [Preparation Example 2] Preparation of Compound (103)
[0126]
[0127] Preparation of compound (206)
[0128] Add 1,2-dibromobenzene (100 g, 423.9 mmol), 2-(9,9′-dimethyl)fluoreneboronic acid (111.0 g, 466.3 mmol), toluene (1000 ml) and tetrakis(triphenylphosphine)palladium (Pd(PPh 3 ) 4 ) (24.5 g, 21.2 mmol), and the mixture was stirred under an argon atmosphere. Aqueous potassium carbonate solution (300 ml) was then added dropwise to the mixture, and the resulting mixture was heated under reflux with stirring for 4 hours. When the reaction was completed, distilled water (1500 mL) was added to the reaction mixture, followed by extraction with ethyl acetate (800 mL). The obtained organic extract was dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. Purification by silica gel column chromatography (ethyl acetate: hexane = 1:30) afforded the product, 1-bromo-2-(9,9'-dimethyl)fluorenylbenzene (75.52 g, 217.0 mmol, yield :...
preparation example 3
[0140] [Preparation Example 3] Preparation of Compound (110)
[0141]
[0142] Preparation of compound (211)
[0143] Add compound (210) (43.90 g, 92.62 mmol) and tetrahydrofuran (1000 ml) in a 500 ml round bottom flask, add n-BuLi (1.6M hexane solution) (55.57 ml, 138.9 ml) dropwise to the flask at -78°C Millimoles). The mixture was stirred at the same temperature for 1 hour, triphenylsilyl chloride (40.95 g, 138.9 mmol) was added dropwise to the reaction mixture, and the temperature was raised to room temperature. The reaction mixture was stirred for 12 hours, and when the reaction was complete, distilled water (1000 ml) was added thereto. The obtained organic extract was extracted with ethyl acetate (800 ml), dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. Purification by silica gel column chromatography (dichloromethane:hexane=1:25) gave compound (211) (34.22 g, 52.33 mmol, yield: 56.5%).
[0144] Preparation of compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com