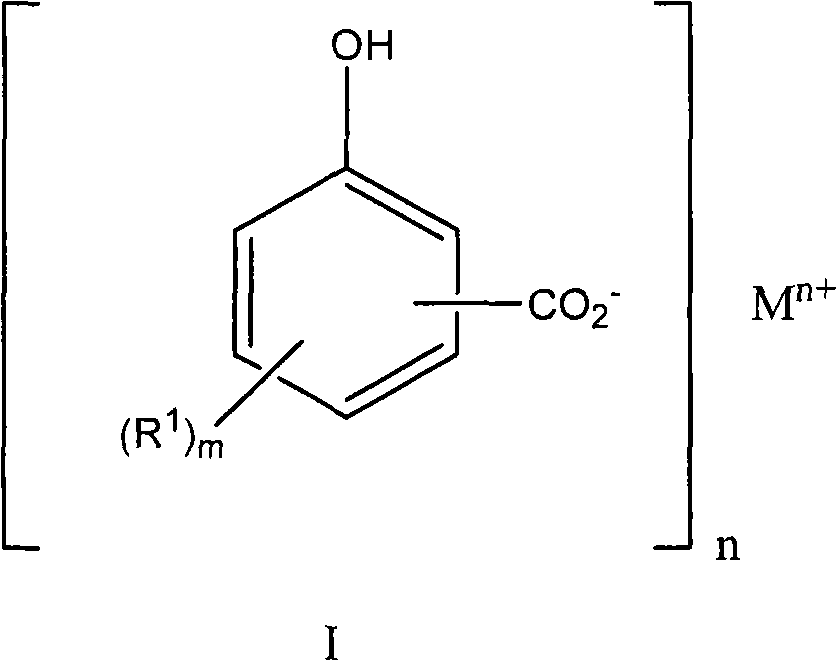
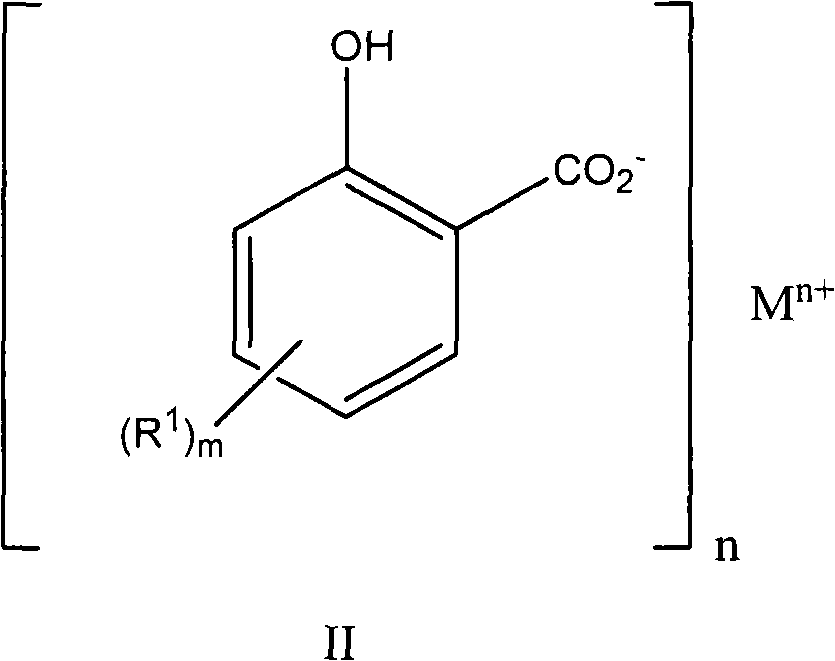
A detergent
A detergent, C10-C40 technology, applied in the direction of additives, carboxylate preparation, carboxylate preparation, etc., can solve problems such as crankcase explosion and reduced effectiveness of engine oil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] Example 1 Preparation of 1-methoxy-4-(hexadecan-1-yl)benzene
[0180] 1-Bromohexadecane (96.32 g) was transferred to a completely dry 2-liter three-necked flask, followed by Li 2 CuCl 4 (0.1M in THF, 31.7ml). Place the reaction flask in an ice / water bath. The reaction was quenched overnight by the dropwise addition of Grignard reagent (4-methoxyphenyl)magnesium bromide (0.5M in THF, 948ml) over two days. The contents of the reaction flask were mixed with toluene (300ml) and poured into a separatory funnel. Then 10% HCl solution was added to acidify the mixture. Water (500ml) was added and shaken with toluene. The aqueous layer was washed with toluene (2 x 300 mL). The organic extracts were combined and washed with water (500ml) and brine (50ml), then washed with MgSO 4 dry. The solvent was removed in vacuo to afford 108.86 g of the title compound as an off-white solid, which was characterized by NMR.
Embodiment 2
[0181] Example 2 Preparation of 4-(hexadecan-1-yl)phenol
[0182] The alkylanisole of Example 1 (40 g) was transferred to a dry 1 liter three-neck flask under nitrogen. Tributylhexadecylphosphonium bromide (12.69 g) and HBr (48% aqueous solution, 71.4 ml) were added thereto. The resulting thick suspension was heated to 135°C and stirred for five hours. Toluene was added and the reaction was transferred to a separatory funnel. The organic layer will be shaken with water, then the aqueous extract will be shaken with fresh toluene. The organic extracts were combined and washed with MgSO 4 dry. The solvent was removed in vacuo to afford the title compound as a brown solid.
Embodiment 3
[0183] Example 3 Preparation of 2-hydroxyl-5-(hexadecan-1-yl)benzoic acid
[0184] 3.1 Phenolization step
[0185] The alkylphenol of Example 2 (52.6 g) was weighed into a 3 liter three-necked flask and xylene (1000 ml) was added using a measuring funnel. Fit the flask for distillation and run 400ml.min on the mixture -1 Nitrogen blanket. Stirring was then started at approximately 400 rpm and the mixture was heated with an oil bath set to 120°C and aqueous sodium hydroxide (50%, 9.53 g) was added dropwise. The temperature was raised to 160°C and all water was removed using a Dean-Stark apparatus. After 4 hours, the reaction was cooled to room temperature overnight.
[0186] 3.2 Carboxylation step
[0187] After cooling, transfer the contents of the flask from step 3.1 above to a 2 L autoclave. A 1 barg (bar gauge) nitrogen mantle was applied, stirring was started and increased to 550 rpm and the autoclave was heated to 138°C. When the autoclave reaches 138 °C, add CO ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| viscosity index | aaaaa | aaaaa |
| viscosity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com