Paracetamol, pseudoephedrine and chlorphenamine compound sustained release preparation
A paracetamol, sustained-release preparation technology, applied in the field of medicine, to achieve the effect of less medication times, stable blood drug concentration, and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
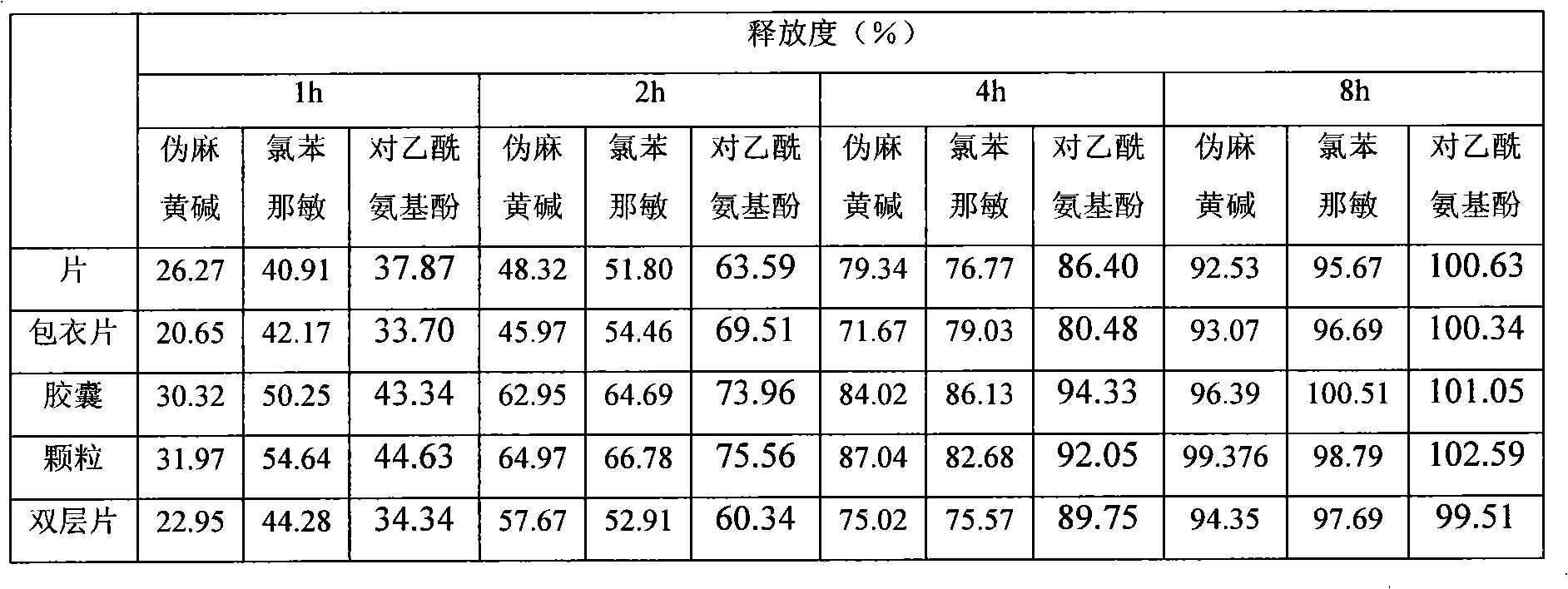
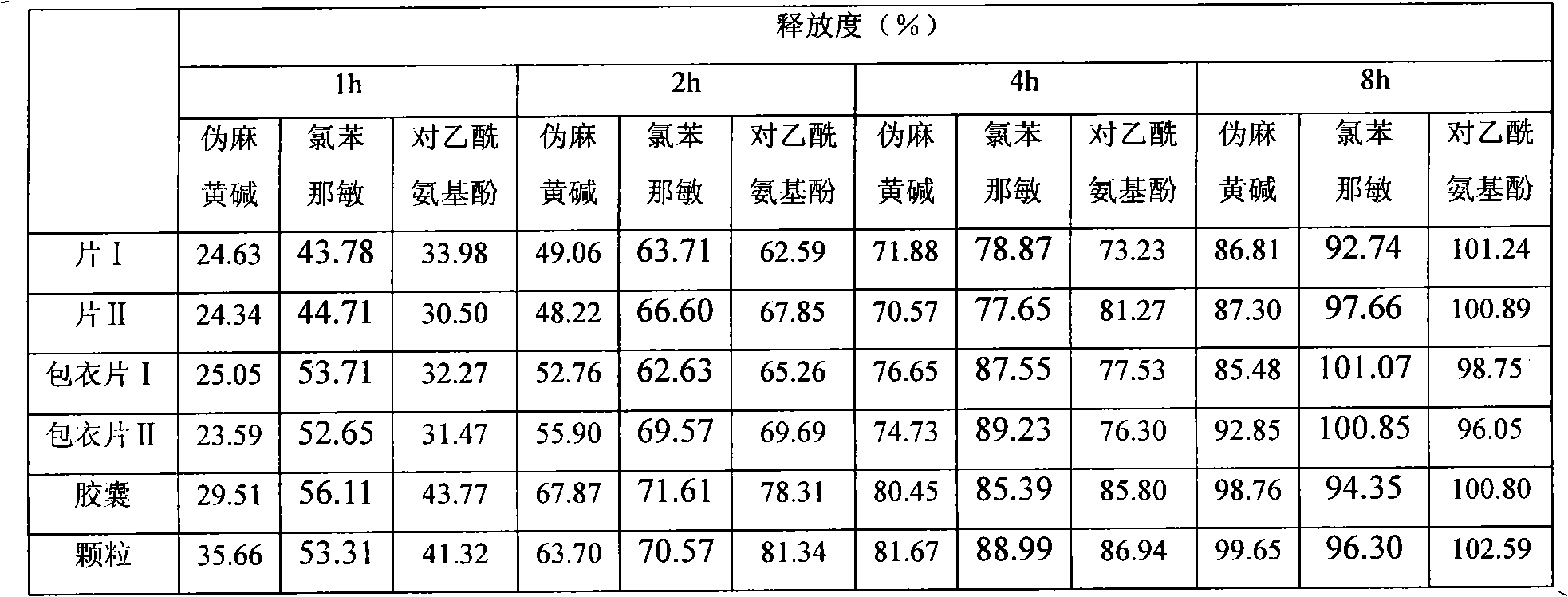
Image
Examples
Embodiment 1
[0020] prescription:
[0021] Acetaminophen 325g
[0022] Pseudoephedrine Hydrochloride 90g
[0023] Chlorpheniramine Maleate 4g
[0024] Hypromellose 40g
[0025] Carnauba Wax 15g
[0026] Microcrystalline Cellulose 36g
[0027] 10% Povidone K 30 Aqueous solution
[0028] Magnesium Stearate Appropriate amount
[0029] Opadry 15g
[0030] Appropriate amount of pure water
[0031]
[0032] Made into 1000 pieces (grains / bag)
[0033] Preparation method 1:
[0034] (1) Preparation of granules Carnauba wax, hydroxypropylmethyl cellulose, and microcrystalline cellulose were respectively passed through an 80-mesh sieve and mixed uniformly. Then add pseudoephedrine hydrochloride, chlorpheniramine maleate, and acetaminophen in turn, mix well, and add 10% povidone K 30 The aqueous solution is a soft material made of an adhesive, wet granules are prepared through a 20-mesh sieve, dried at 50°C, granulated through a 20-mesh sieve, a...
Embodiment 2
[0050] prescription:
[0051] Acetaminophen 325g
[0052] Pseudoephedrine Hydrochloride 90g
[0053] Chlorpheniramine Maleate 4g
[0054] Hypromellose K100M 30g
[0055] Hypromellose E5 75g
[0056] Microcrystalline Cellulose 45g
[0057] 10% Copovidone K 30 Aqueous solution
[0058] Appropriate amount of stearic acid
[0059] Opadry 50g
[0060] Appropriate amount to completely dissolve in water
[0061]
[0062] Made into 1000 pieces (grains / bag)
[0063] Preparation method 1:
[0064] (1) Preparation of granules: Hypromellose and microcrystalline cellulose are passed through 80-mesh sieves respectively, fully mixed with equal increment method, then add pseudoephedrine hydrochloride, acetaminophen, maleic acid chloride pheniramine 2g, make it mix well, take 10% copovidone K 30 The aqueous solution is a soft material made of adhesive, wet granules are prepared through a 20-mesh sieve, dried at 60°C, granulated through a 20-m...
Embodiment 3
[0082] prescription:
[0083] pseudoephedrine sulfate
90g
2g
325g
sodium alginate
60g
58g
50g
10% Povidone K 25 aqueous solution
Appropriate amount
Appropriate amount
[0084] Coating prescription:
[0085] pseudoephedrine sulfate
30g
2g
Opadry
50g
[0086] ethanol
Appropriate amount until completely dissolved
[0087] Made into 1000 pieces (grains / bag)
[0088] Preparation:
[0089] (1) Preparation of granules Sodium alginate, gelatin, and pregelatinized starch were respectively passed through a 100-mesh sieve, fully mixed in an equal increment method, and then added pseudoephedrine sulfate, chlorpheniramine maleate, and para-acetamide Phenol, mixed well with 10% Povidone K 25 The aque...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com