Hapten and antigen of nitrofurans and preparation method and application thereof
A technology of nitrofuran and substances, which is applied in the field of haptens and antigens of nitrofuran substances and their preparation and application. It can solve the problems of complex pretreatment, high technical requirements for operation, and long operation time, and shorten the detection time. , Improve detection efficiency and reduce detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
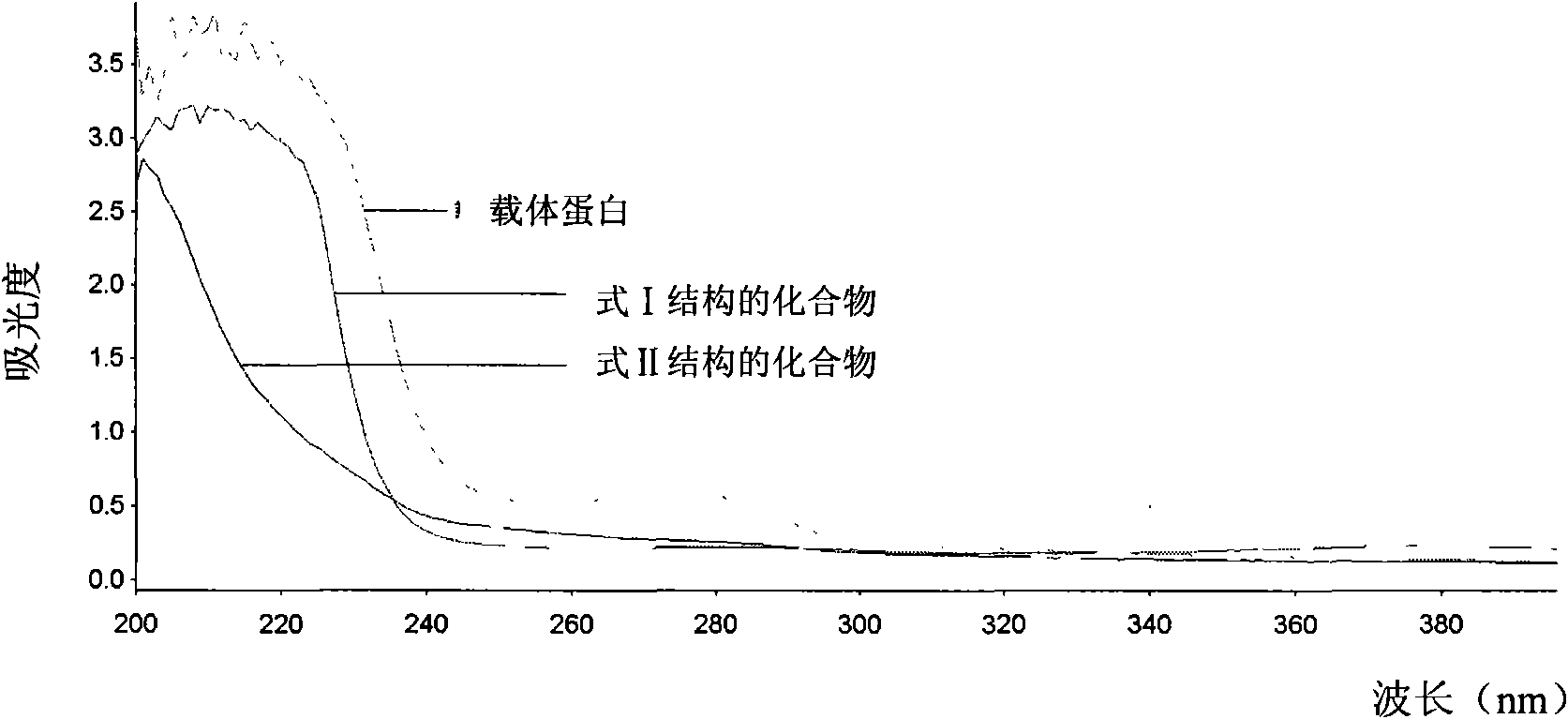
Image
Examples
Embodiment 1
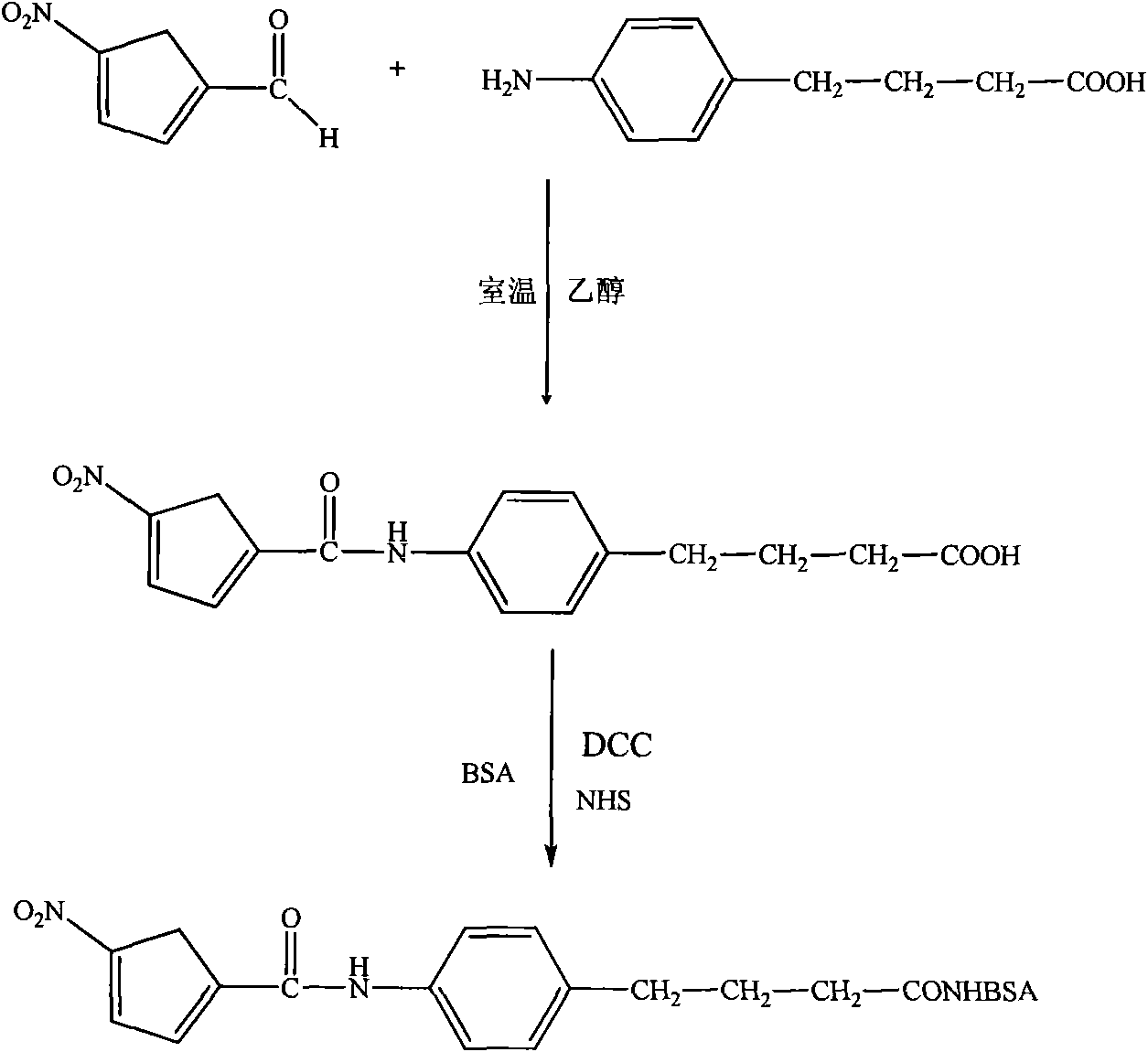
[0040] Synthesis and identification of embodiment 1, formula I structure compound
[0041] 1, the synthesis of formula I structure compound
[0042] 1) Dissolve 139mg (1mmol) of 5-nitrofuran-2-carbaldehyde in an aqueous solution of ethanol consisting of 1mL of pure water and 1mL of absolute ethanol, and magnetically stir to completely dissolve it;
[0043] 2) Dissolve 179mg (1mmol) p-aminophenylbutyric acid in 1mL absolute ethanol;
[0044] 3) Mix the solution of the above step 1) and step 2), the color of the mixture gradually turns yellow, after stirring for 1 minute, a yellow precipitate appears, continue to stir at room temperature, overnight, and centrifuge to obtain a yellow precipitate; dry the precipitate at 60°C , 223mg of product was obtained by weighing, the yield was 71%.
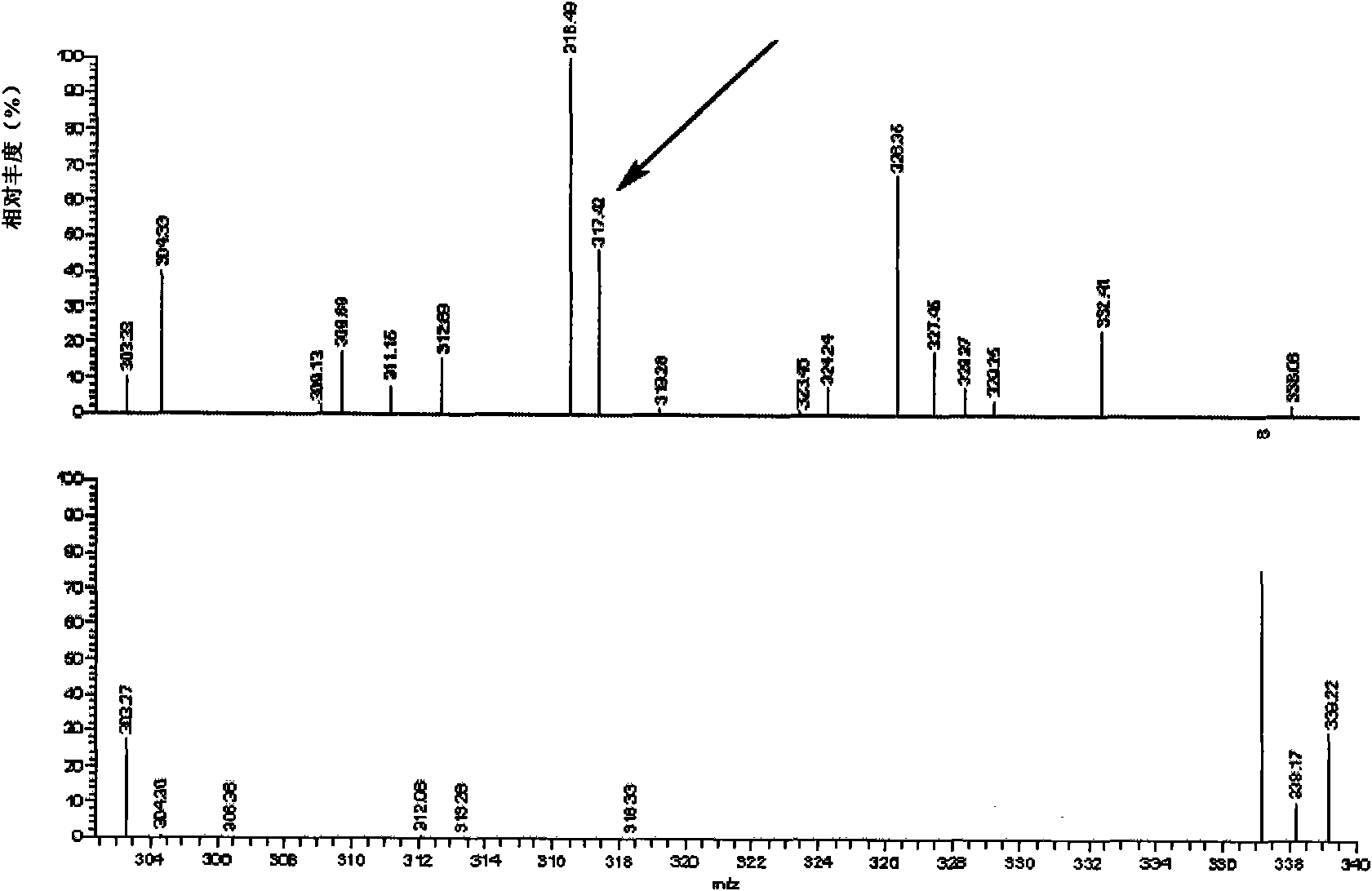
[0045] 2. Identification of the compound of formula I
[0046] The molecular structure of the compound obtained in the above step 1 was determined by ESI. The result is as figure 2 shown. ...
Embodiment 2
[0050]Embodiment 2, the synthesis and identification of nitrofuran compound
[0051] 1. Synthesis of nitrofuran compounds
[0052] 1) Dissolve 15 mg of the compound of the formula I prepared in Example 1 above in 1 mL of N,N-dimethylformamide, and magnetically stir to completely dissolve it;
[0053] 2) Add 15 mg DCC and 15 mg NHS to the solution of the above step 1) respectively, react at room temperature overnight, and centrifuge to remove the precipitate;
[0054] 3) 50 mg of bovine serum albumin (BSA) was dissolved in 5 mL of 0.1 mol / L sodium carbonate solution of pH 9.6;
[0055] 4) Add the solution of the above step 3) dropwise to the solution of the above step 2), and stir and react for 4 hours;
[0056] 5) Put the reaction solution of the above step 4) into a dialysis bag, dialyze with a normal saline solution for 72 hours at 4°C, and change the dialysate 6 times during this period; pour out the dialysate, and put the solution in the dialysis bag under sterile condit...
Embodiment 3
[0059] Embodiment 3, the preparation of antibody
[0060] 1. Preparation of antiserum from immunized animals
[0061] Five New Zealand white rabbits were used as immunized animals, and the nitrofuran compound prepared in the above-mentioned Example 2 was used as the immunogen, and the immunizing dose was 1 mg / kg. The immunization method is as follows: For the first immunization, fully emulsify the immunogen with an equal volume of Freund’s complete adjuvant, inject it subcutaneously at multiple points on the back of the neck, and mix the immunogen with an equal volume of Freund’s incomplete adjuvant to prepare into an emulsifying agent, booster immunization once, a total of 8 times of immunization (including a total of 8 times of booster immunization), and no adjuvant was added for the last time.
[0062] The rabbits were killed 7-10 days after the last immunization, and blood was collected by heart blood collection. Each rabbit can collect about 80 mL of blood. Place the col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com