Method for preparing sustained-release microspheres containing micronized recombinant human vascular endothelial inhibin
A technology of vascular endothelium and slow-release microspheres, which is applied in the directions of medical preparations, drug combinations, and pharmaceutical formulations containing active ingredients, can solve the problems of not widely used, large proportion of excipients, large protein particles, etc., and can improve the emulsification efficiency. , Improve the encapsulation efficiency and reduce the loss of protein activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
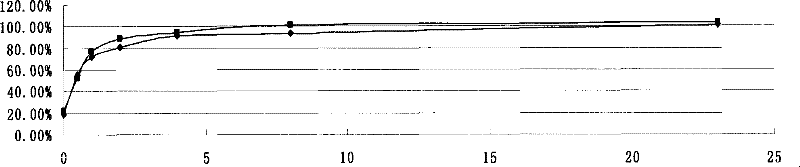
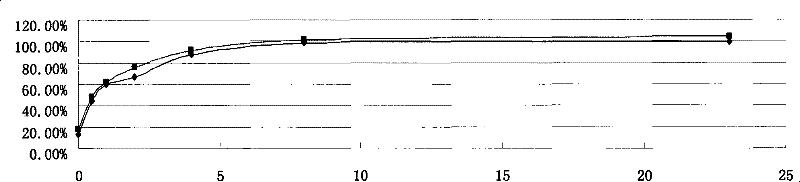
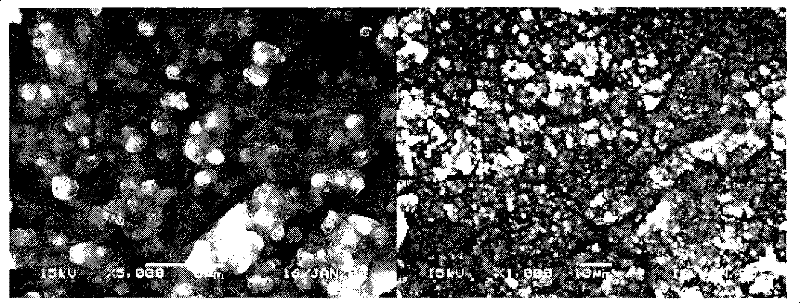
[0049] Accurately weigh 240 mg of recombinant human endostatin Endostar micropowder (containing 200 mg of recombinant human endostatin Endostar), dissolve 400 mg of PLGA (Mw=48000, 50:50) in 10 ml of acetonitrile, add the micropowder into PLGA dichloromethane In the mixed organic solution with acetonitrile, dichloromethane:acetonitrile=1:4 (volume ratio). 4000rpm high-speed dispersion and emulsification to form S / O colostrum. Pour the above S / O colostrum into soybean oil containing 0.3% lecithin and 0.1% sucrose ester, mechanically stir the volatile solvent (1000rpm) for 4 hours, use a 0.8 μm organic microporous membrane to filter to obtain microspheres, and use petroleum Washed three times with ether, and finally freeze-dried to obtain the finished microspheres. The finished microspheres were dissolved in dichloromethane, and the drug was extracted with a PBS solution with pH=7.4. The protein concentration in the extracted solution was determined by HPLC to obtain the drug l...
Embodiment 2
[0052] Accurately weigh 120 mg of recombinant human endostatin Endostar micropowder (containing 100 mg of recombinant human endostatin Endostar), dissolve 400 mg of PLGA (Mw=10000, 50:50) in 10 ml of acetonitrile, and add the micropowder into the acetonitrile solution of PLGA , 4000rpm high-speed dispersion and emulsification to form S / O colostrum. Pour the above S / O colostrum into the medicinal light liquid paraffin containing 0.5% Span 80, mechanically stir the volatile solvent (500rpm) for 4 hours, use a 0.8 μm organic microporous membrane to filter to obtain microspheres, and use petroleum Washed three times with ether, and finally freeze-dried to obtain the finished microspheres. The finished microspheres were dissolved in dichloromethane, and the drug was extracted with a PBS solution with pH=7.4. The protein concentration in the extracted solution was determined by HPLC to obtain the drug loading and encapsulation efficiency of the microspheres. The drug loading is 28....
Embodiment 3
[0055] Accurately weigh the same batch of micronized Endostar protein used in Example 1, redissolve with buffer, and dilute to 1500ng / ml, 1200ng / ml, 1000ng / ml, 800ng / ml, 600ng / ml, 400ng / ml, 200ng / ml, 100ng / ml, 50ng / ml. The immunological activity was measured with a commercially available ELISA (enzyme-linked immunoassay) kit. Method: Make a standard curve of unmicronized protein, and then make a standard curve of reconstituted micronized protein, compare the difference in absorbance value (OD) between the two curves at 100ng / ml-1000ng / ml, and obtain the immunological activity retention Rate. The calculation method of activity retention rate is: activity retention rate=100%-(non-micronized protein OD-micronized protein OD) / (non-micronized protein OD-blank value 0.0392)*100%. Table 1 shows that the protein activity retention rate after micronization is greater than 90%.
[0056] Table 1
[0057]
[0058]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com