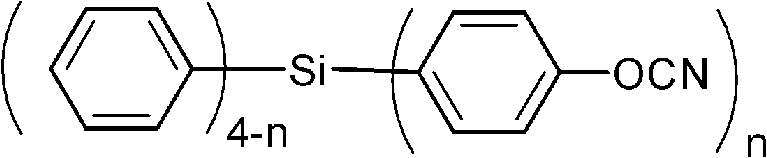Aromatic cyanate ester monomer containing silicon and preparation method thereof
A technology for aromatic cyanate ester and cyanate ester is applied in the field of cyanate ester monomer and its preparation, which can solve the problems of reducing key physical properties and having no cyanate ester compound with aryl silicon structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] Synthesis of bis(4-bromophenyl)disilane: Dissolve 25g of 1,4-p-dibromobenzene in 80ml of tetrahydrofuran, cool down to -78°C, and slowly add 31.2ml of 2.5N n-butyllithium n-hexane solution dropwise After the dropwise addition was completed and stirring was continued for 0.5 hours, 2.4 ml of diphenyldichlorosilane was added dropwise, and after the dropwise addition was completed, the reaction was continued for 5 hours, and the reaction was terminated with dilute hydrochloric acid aqueous solution. The organic phase was separated, dried with anhydrous magnesium sulfate, and the solvent was spin-dried, and the residue was recrystallized with ethyl acetate to obtain 14 g of white crystals with a yield of 87.4%.
[0024] Synthesis of bis(4-hydroxyphenyl)disilane: Dissolve 1.9g of bis(4-bromophenyl)disilane in 40ml of tetrahydrofuran, cool to -78°C, slowly add dropwise 3.7ml of 2.5N n-butyllithium n-hexane solution, after the dropwise addition, add 3.5ml of trimet...
Embodiment 2
[0029]
[0030] Synthesis of tris(4-bromophenyl)phenylsilane: Dissolve 18g of 1,4-p-dibromobenzene in 80ml of tetrahydrofuran, cool to -78°C, slowly add 47.4ml of 2.5N n-butyllithium in n-hexane After the solution was continuously stirred for 4 hours after the dropwise addition, 1.5 ml of phenyltrichlorosilane was added dropwise. After the dropwise addition, the reaction was continued for 2 hours, and the reaction was terminated with dilute hydrochloric acid aqueous solution. The organic phase was separated, dried with anhydrous magnesium sulfate, and the solvent was spin-dried, and the residue was recrystallized with dichloromethane to obtain 2 g of white crystals with a yield of 89.2%.
[0031] Synthesis of tris(4-hydroxyphenyl)phenylsilane: Dissolve 2.3g of tetrakis(4-bromophenyl)silane in 50ml of tetrahydrofuran, cool to -78°C, slowly add 9.3ml of 2.5N n-butyl lithium dropwise n-hexane solution, after the dropwise addition, add 2.5ml of triisopropyl borate dropwise, con...
Embodiment 3
[0036]
[0037] Synthesis of tetrakis(4-bromophenyl)silane: Dissolve 25g of 1,4-p-dibromobenzene in 80ml of tetrahydrofuran, cool to -78°C, slowly add 63.2ml of 2.5N n-butyllithium n-hexane solution dropwise, After the dropwise addition was completed and stirring was continued for 1 hour, 2.5 ml of tetrachlorosilane was added dropwise, and the reaction was continued for 2 hours after the dropwise addition, and the reaction was terminated with dilute hydrochloric acid aqueous solution. The organic phase was separated, dried with anhydrous magnesium sulfate, and the solvent was spin-dried, and the residue was recrystallized with dichloromethane to obtain 16 g of white crystals with a yield of 93.7%.
[0038] Synthesis of tetrakis(4-hydroxyphenyl)silane: Dissolve 1.3g of tetrakis(4-bromophenyl)silane in 50ml of tetrahydrofuran, cool to -78°C, slowly add 7.3ml of 2.5N n-butyl lithium in n-hexane Solution, after the dropwise addition, add 3.5ml trimethyl borate dropwise, continu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com