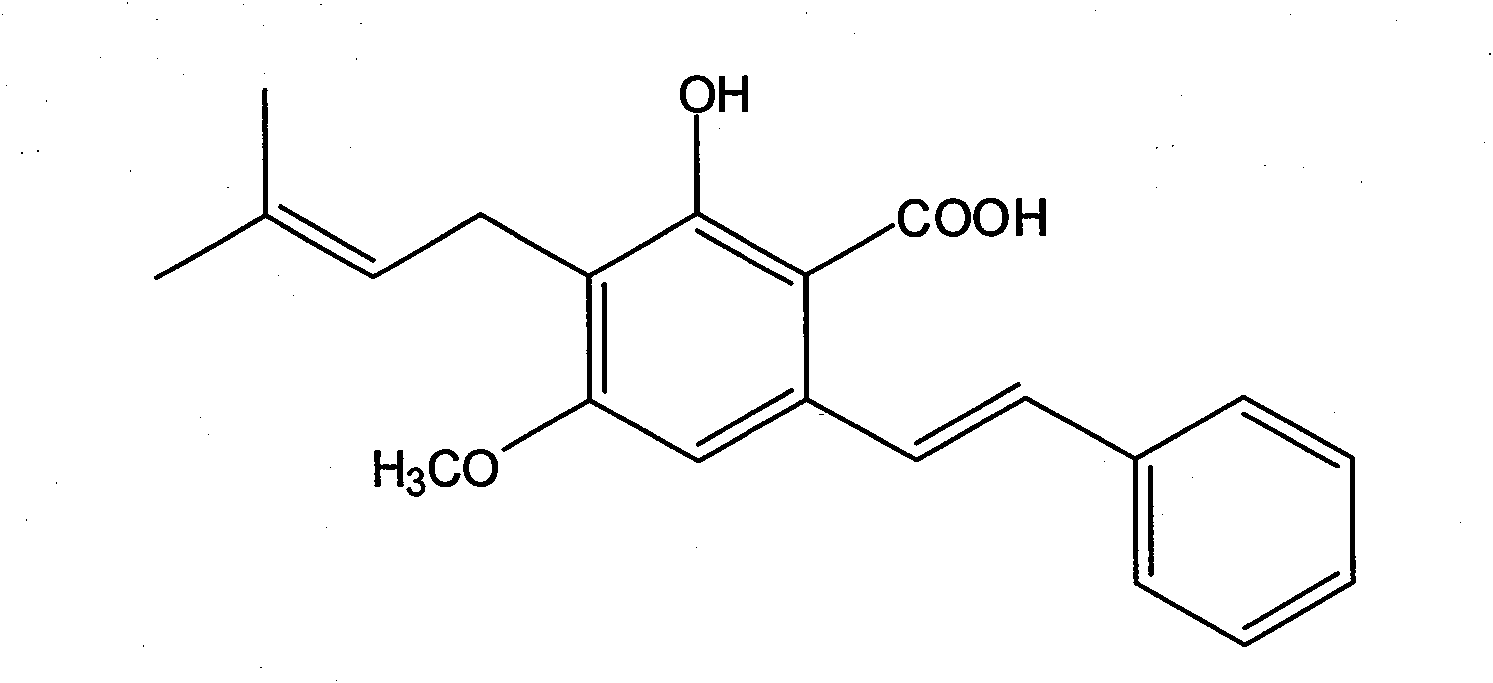
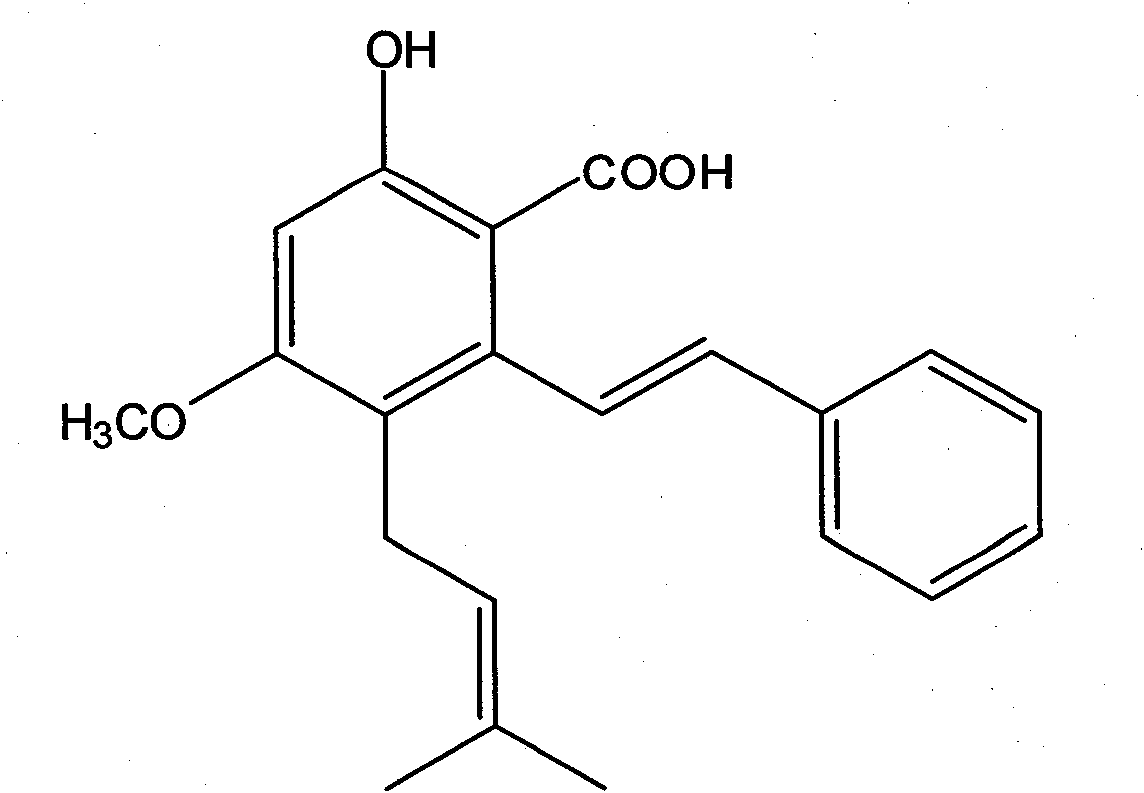
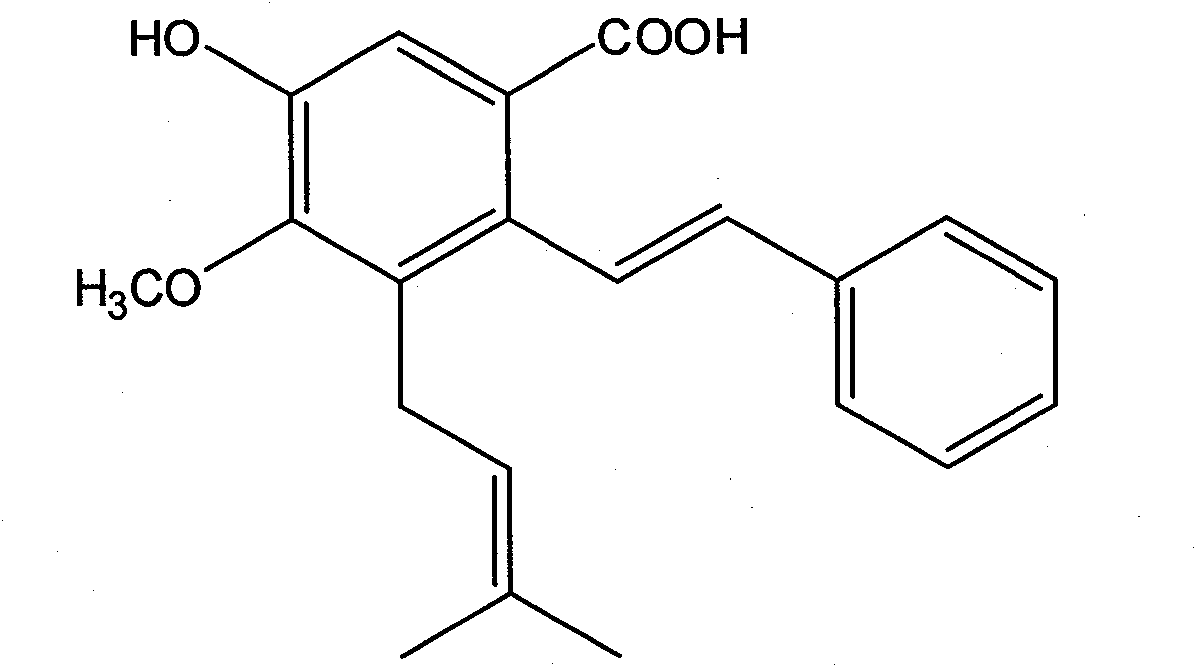
Application of pigeon pea stilbene acid in the preparation of antitumor drug
A tumor drug, pigeonpea leaf technology, applied in the field of medicine, to achieve the effect of low toxicity, not easy to produce drug resistance, efficient and rational use, and good anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0016] Cells: human breast cancer cells (MCF-7), human lung cancer cells (A549), human gastric cancer cells (SGC-7901), human colon cancer cells (HCT-8), human leukemia cells (CEM), human ovarian cancer cells ( HO-4980), human cervical cancer cells (Hela), human liver cancer cells (HepG2), human glioma cells (SHG-44), mouse solid tumor S180 cells. All were purchased from Shanghai Cell Bank.
[0017] Experimental materials: pigeonpea supercritical extract and pigeontostilnic acid, including pigeontostilnic acid Ⅰ-Ⅵ, prepared in our laboratory; normal saline, Harbin Sanlian Pharmaceutical; paclitaxel, Shanghai Haohua Chemical Co., Ltd.
[0018] method:
[0019] (1) Tumor cells in the exponential growth phase were collected and diluted to 20,000 cells / mL with RPMI1640 culture medium containing 10% fetal bovine serum.
[0020] (2) Add 200 μL of cell suspension to each well of a 96-well plate and place at 37°C, 5% CO 2 Cultivate in the incubator for 24h.
[0021] (3) Dissolve ...
example 2
[0033] S180 ascites subcultured mice were purchased from Heilongjiang Cancer Research Hospital. Kunming mice, weighing 24-25 g, were purchased from the Experimental Animal Center of Heilongjiang Cancer Hospital. Cyclophosphamide was purchased from the Second Affiliated Hospital of Harbin Medical University.
[0034] Methods: Experimental mice were randomly divided into control group (normal saline 200mL / kg), cyclophosphamide (20mg / kg), pigeontostilnic acid I (40mg / kg, 15mg / kg), pigeonpea supercritical extract ( 250mg / kg, 125mg / kg) due to the decomposition of paclitaxel after intragastric administration, cyclophosphamide was selected as the positive control drug for the in vivo test. Select mice with tumor transplantation for 7 days, good tumor growth and obvious abdominal bulge, disinfect the abdominal skin, use a disposable non-toxic blood sampling device to penetrate the abdominal cavity through the abdominal wall, extract ascites, put it into a sterile beaker and dilute it...
example 3
[0039] 1. Using laser confocal microscope to study the DNA morphology of breast cancer MCF-7 cells
[0040]Take MCF-7 cells in the logarithmic growth phase, add 5 μM pigeontostilnic acid, and set a negative blank control. MCF-7 cells were incubated for 48 hours, culture medium was removed, washed twice with cold PBS, PC-3 cells were digested with 0.25% trypsin, and MCF-7 cells were collected by centrifugation at 3000×g, washed twice with cold PBS, and centrifuged at 3000×g 5min to remove PBS. The cells were collected by centrifugation at 3000×g for 5 minutes, and resuspended with cold PBS buffer to adjust the cell density to 1×10 3 / mL, and add 1mL 50μg / mL DAPI, pipette evenly, incubate at room temperature in the dark for 5min, and use a laser confocal microscope to study the DNA morphology of prostate cancer MCF-7 cells.
[0041] result: figure 2 (A) is the blank control MCF-7 cells, figure 2 (B) is the MCF-7 cells added with drugs. In comparison, (B) the nuclear chroma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com