Antihypertensive drug cilazapril intermediate and preparation method thereof
A cilazapril and antihypertensive technology, which is applied in the field of preparing antihypertensive drug cilazapril intermediates, can solve the problems of unsuitability for industrialized production, complicated column-passing process, and high three-waste discharge, and avoids the need for column-passing. Split, simplify the synthesis process, reduce the effect of the three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
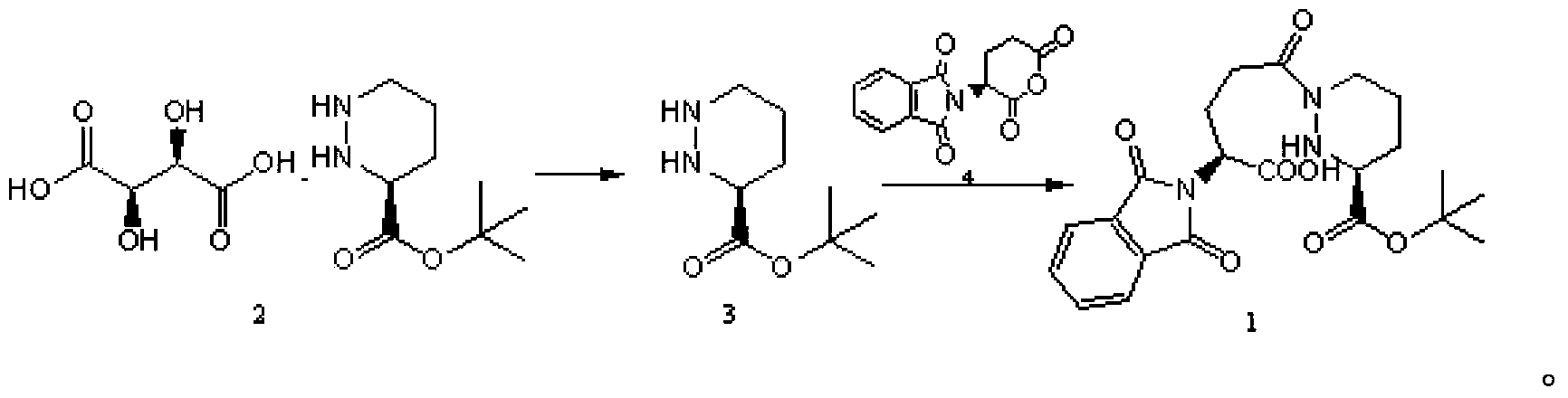
[0018] The preparation of the compound N-phthaloylamino-L-glutamic anhydride of structural formula 4, concrete reaction formula is as follows:
[0019]
[0020] 20 g of L-glutamic acid and 31.9 g of N-ethoxycarbonylphthalic imide were sequentially added to 360 ml of an aqueous solution containing 28.8 g of sodium carbonate. Stir at room temperature, then extract with ethyl acetate. After the water layer was cooled, the water phase was adjusted to pH=2 with hydrochloric acid, then extracted with ethyl acetate, washed with saturated sodium chloride solution, dried, filtered, and concentrated under reduced pressure to obtain an oily product, which was recrystallized from water to obtain a white solid product N - Phthalylamino-L-glutamic acid 19.0g Melting point: 153-154°C
[0021] 18.5 g of N-phthaloylamino-L-glutamic acid was dissolved in 53.1 g of acetic anhydride, and heated to 110° C. for 5 minutes. Concentrate and add ether. After filtration, 16.5 g of white solid prod...
Embodiment 2
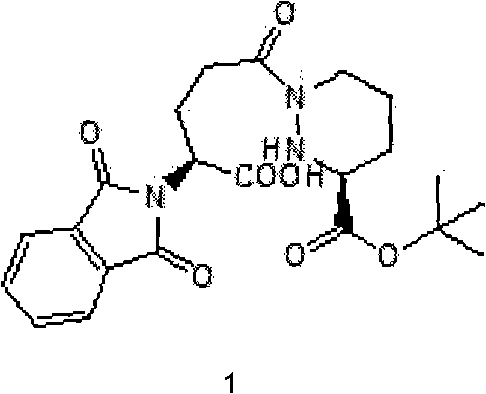
[0023] The compound of structural formula 1 (2S)-5-[(S)-3-(tetrahydro-3-tert-butoxycarbonyl)pyridazinyl-1-yl]-2-(1,3-dioxo-2H- Preparation of isoindol-2-yl)-5-oxopentanoic acid
[0024] Example 2
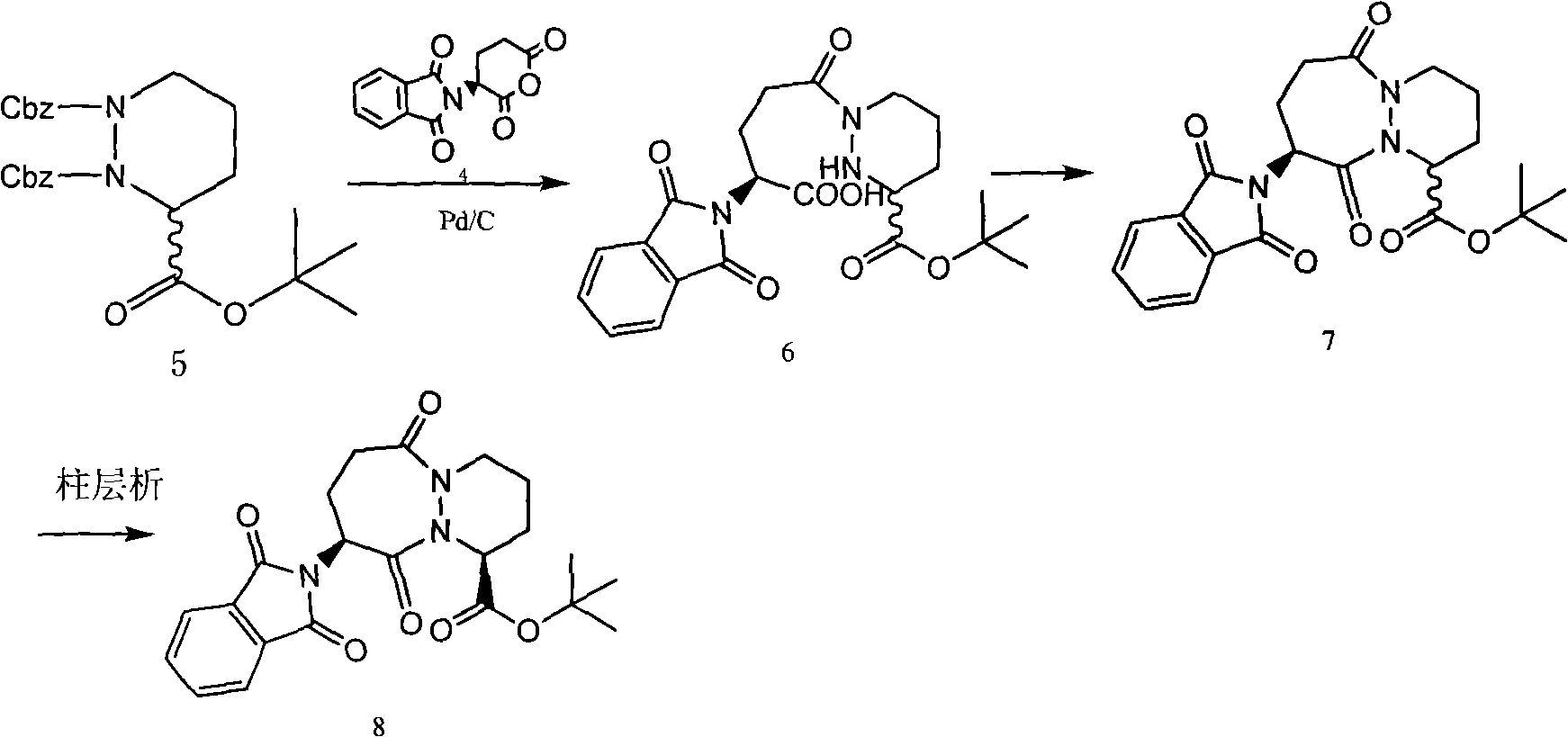
[0025] Add 9.2g of the compound of structural formula 2 to 40ml of tetrahydrofuran, stir to form a suspension solution, slowly drop into 140ml of 5% sodium bicarbonate solution to alkalinize and remove L-type tartaric acid, then drop into 30ml of tetrahydrofuran solution of 7.1g of the compound of structural formula 4, at room temperature After stirring for half an hour, after the reaction was complete, the aqueous layer was extracted with 30ml of ethyl acetate. The extracted aqueous layer was cooled to 0-5°C, and the pH value was adjusted to 3 with concentrated hydrochloric acid. A white solid was precipitated, and 10.8g of compound 1 was obtained after filtration. MS: (M+Na) + =468 +
Embodiment 3
[0027] Dissolve 33.5g of sodium bicarbonate in 350ml of water, drop into 150ml of tetrahydrofuran solution of 44.8g of compound of structural formula 2, then add 50ml of tetrahydrofuran solution of 34.5g of compound of structural formula 4, stir at room temperature for one hour, extract water with 60ml of ethyl acetate layer, the extracted water layer was cooled to 0-5°C, and the pH value was adjusted to 3.5 with concentrated hydrochloric acid, a large amount of white solid was precipitated, and 55.5 g of compound 1 was obtained after filtration. MS: (M+Na) + =468 +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com