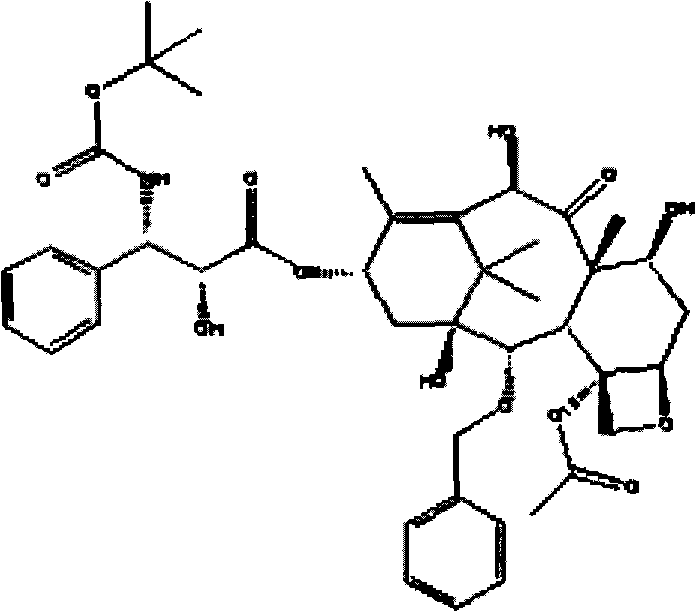
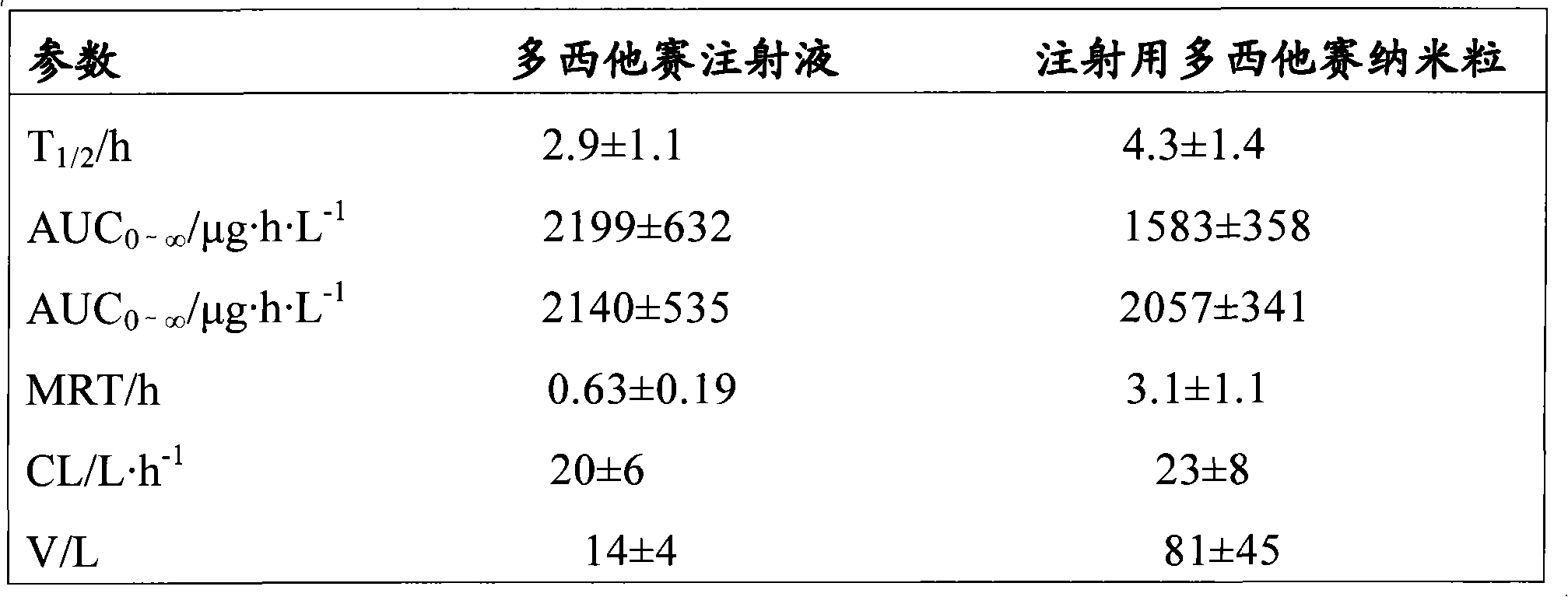
Docetaxel nanometer lipid injection, preparation method and purpose thereof
A nano-lipid, docetaxel technology, applied in the field of medicine, can solve the problems of hemolysis, allergy, and complicated use methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1, formula and preparation method of docetaxel nano lipid preparation of the present invention
example
[0059] Table 1: Typical prescription of docetaxel nano lipid preparation of the present invention
[0060] Ingredient name Weight ratio Function in formulation
[0062] Phospholipid 10-20 Emulsifier
[0063] 1,2-Propanediol 10-20 Stabilizer, co-surfactant
[0064] Polyethylene glycol-12-hydroxystearate 40~60 emulsifier, co-stabilizer
[0065] Absolute Ethanol 10-30 Solvent
[0066] Glycerol (glycerin) 10-30 stabilizer, co-surfactant
[0067] Pretreatment of raw and auxiliary materials for each component in the above prescription → batching according to the ratio in the above prescription → high-speed stirring → filtration → excipient liquid preparation → sterile press filtration → filling → plugging → capping → labeling → Light inspection→packaging→storage after passing the inspection.
Embodiment 2
[0068] The performance detection test of the docetaxel nano-lipid preparation of embodiment 2, embodiment 1
[0069] The docetaxel nano-lipid preparation of Example 1 of the present invention is a light yellow transparent solution, which can be mixed with water for injection in any proportion, and the formed mixed solution has opalescence. Using transmission electron microscope and laser particle size analyzer to study its morphology, it shows that the particle size distribution of this product is narrow, and the nanoparticles with a particle size of 10-200nm are more than 90%, with round particle size and good dispersion; free docetaxel is analyzed by HPLC method According to the test, the drug loading of nanoparticles is greater than 98%; this product is placed in a cool and dry place for 6 months, the content change is less than 2%, and the related substances are less than 1%, which initially shows that this product has good stability; this product is compatible with infus...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com