Lamivudine diastereoselective synthesis method
A technology of lamivudine and synthetic method, which is applied in the direction of drug combination, digestive system, organic chemistry, etc., can solve the problems that are not conducive to the health of operators, strong triethylamine, expensive raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
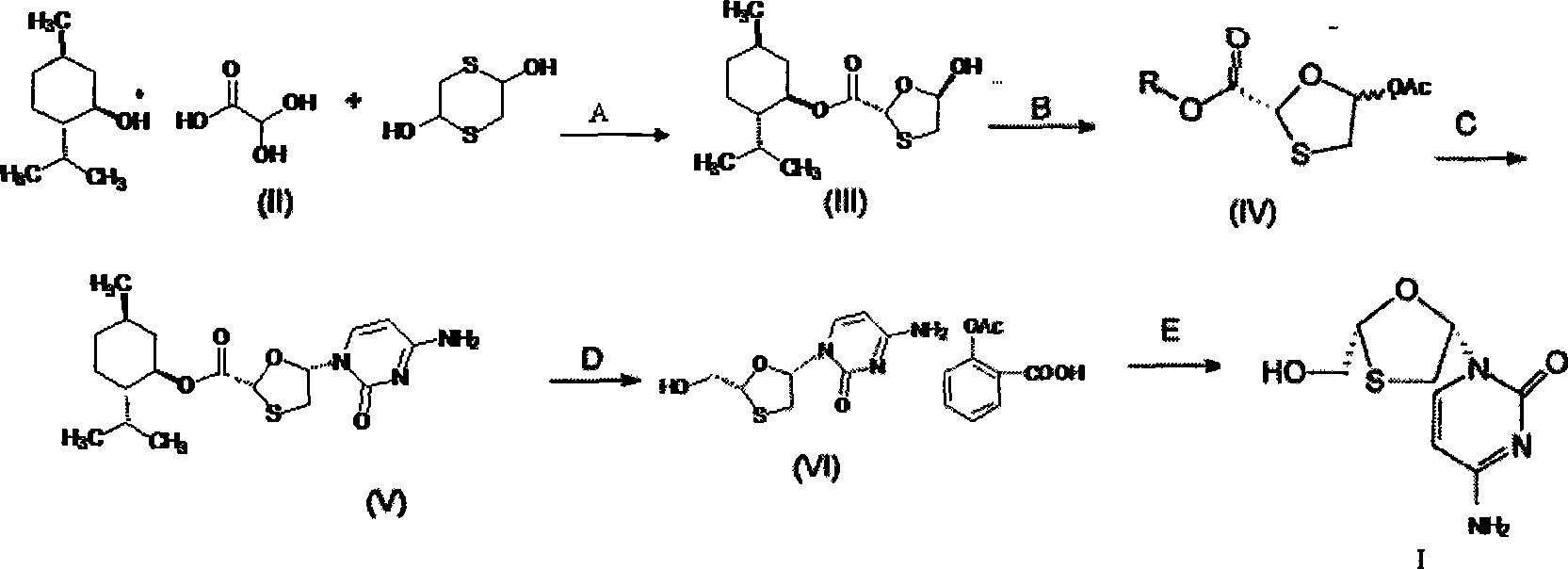
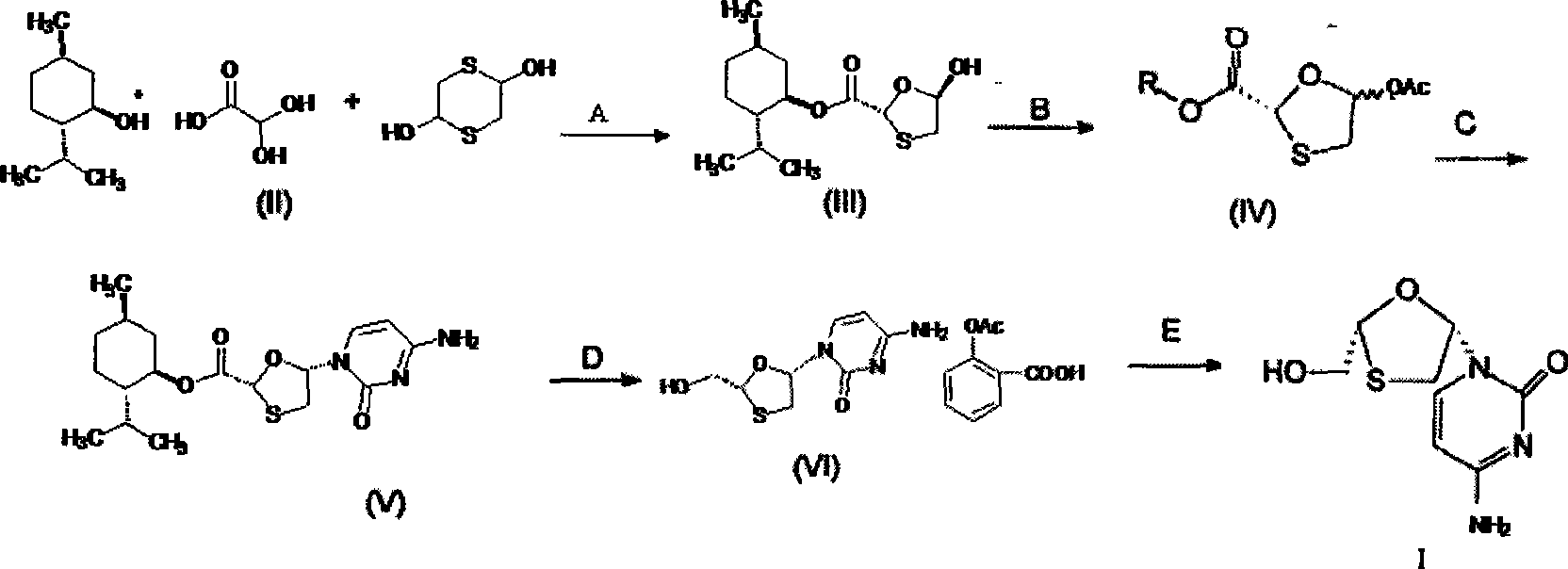
[0050] A preparation method of lamivudine. Its production method is:
[0051] step 1:
[0052] Preparation of trans 5-hydroxyl-1,3-oxathiolane-2-carboxylic acid-(1'R, 2'S, 5'R) menthol ester (i.e. intermediate III):
[0053]
[0054] In a 500mL three-necked flask, add 17.5mL (0.1mol) L(-) menthol, 50% 18.4g (0.1mol) glyoxylic acid monohydrate, 200mL isopropyl ether and 3mL concentrated sulfuric acid, reflux reaction under vigorous stirring After 10 hours, change the condenser tube to a water separator, and slowly separate a certain amount of water. After cooling slightly, add 15.2g (0.1mol) of 2,5-dihydroxy-1,4-dithiane, and the mixture is refluxed for 3 hours , cooled, filtered with suction, the filtrate was concentrated under reduced pressure to 100mL and then cooled at 0-5°C, slowly added 2mL of triethanolamine in 200mL n-heptane solution, stirred at the same temperature for 8h, filtered with suction, and the filter cake was washed with a small amount of isopropyl Was...
Embodiment 2
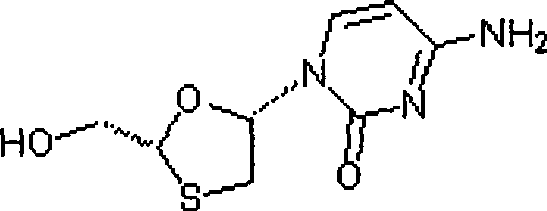
[0072] The other steps of Example 2 are the same as in Example 1, except that 4-amino-1-(2R-hydroxymethyl-[1,3]oxathiolane-5S-yl)-1H-pyrimidine in step 4 The preparation of -2-ketone monoaspirin salt (i.e. intermediate VI) is as follows:
[0073] Add 38.1g (0.1mol) 5S-cytosine-1'-yl-1,3-oxathiolane-2-carboxylic acid-(1'R, 2'S, 5'R) to a 500mL three-necked flask Menthyl ester (i.e. intermediate V), 200mL tetrahydrofuran, under stirring, add 5.4g (0.1mol) potassium borohydride and 4.2g (0.1mol) lithium chloride, stir at room temperature for 15h, and slowly add 10mL of 10% dilute Hydrochloric acid, stirred for 10 min, filtered, the filtrate was evaporated to dryness under reduced pressure, the residue was added to 200 mL of water, the aqueous layer was extracted with isopropyl ether (100 mL x 3) to remove menthol, 19.8 g (0.11 mol) of aspirin was added, and heated to 70 ° C to obtain The solution was clarified, placed in the refrigerator for crystallization, filtered, and dried ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com